
�ɶ����ڳ���Ԫ���γɵĴ�����A��B��C��D��E��F��Xת����ϵ����ͼ��ʾ��ij��������ȥ����
��֪��B��XΪ���ʣ�������DΪ��ɫҺ�壬A��B��ͬһ��Ԫ�ء�
��ش��������⣺
��1����E�����Ǵ�����Ⱦ�F��һԪǿ�ᡣ
��д��E��F��Ӧ�Ļ�ѧ����ʽ�� ��
������25��ʱ0.1 mol��L��1 A��ˮ��Һ���������м�������0.1 mol��L��1��ϡ���ᣬ��������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳���� ��
���ڳ����£���V1 L pH��a��A��ˮ��Һ�м���V2 L pH��b�����ᣬ��a��b��14����ǡ����ȫ��Ӧ����V1��V2�Ĺ�ϵΪVl V2���������������������������ȷ����������pH��������Һ������Ƚϣ���ˮ�������c(H��)ǰ��Ϊ���ߵ�108������������Һ��pH�� ��
��2����E���岻�Ǵ�����Ⱦ�F�Ƕ�Ԫ���ᡣ
��B����Ԫ�������ڱ��е�λ�� ��
��д����������C��Ӧ�Ļ�ѧ����ʽ�� ������������Eͨ������������Һ�еò�����F��F��Ksp��2.8��10��9���ֽ��ó�������0.1 mol��L��1��CaCl2��Һ�У���Ksp �����������С�����䡱������ʱ����ɲ�����F������������Һ�е�Ũ��Ϊ ��
��1���� 3NO2+H2O=2HNO3+NO ��c(H+)> c( SO42-) > c(NH4+) >c(OH��) �� �� 3
��2���ٵڶ����ڢ�A�� �� Fe2O3+3CO 2 Fe +3CO2 ���� 2.8��10-8 mol��L-1
2 Fe +3CO2 ���� 2.8��10-8 mol��L-1
�������������(1)�������ʼ���ת����ϵ����֪��������֪A��NH3��B��N2��C��NO��D��H2O��E��NO2��F��HNO3��X��O2����д��E��F��Ӧ�Ļ�ѧ����ʽ��3NO2+H2O=2HNO3+NO���ڰ�ˮ������������Ũ�Ȼ�ϵõ���ΪNH4HSO4��Һ��NH4HSO4= NH4++H++SO42-�����ڷ�����NH4++H2O  NH3��H2O+H+��H2O
NH3��H2O+H+��H2O OH-+H+������������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����c(H+)> c( SO42-) > c(NH4+) >c(OH��)�����ڳ����£�����NH3��H2O pH��a,C(H+)= 10-amol/L ,C(OH-)= 10(a-14)mol/L pH��b�����ᣬC(H+)=10-bmol/L ��a��b��14������C(H+)= C(OH-)�����ڼ�Ϊ����C(NH3��H2O)>C(HCl).��ǡ����ȫ��Ӧ�� V1��V2�Ĺ�ϵ��V1��V2����NH4Cl��HCl��PH��ȣ��������ǵ�PHΪX����ǰ��ˮ���������C(H+)=10-amol/L,������ˮ�������c(H��)Ϊ10-(a+8)mol/L, C(OH-)= c(H��)=10-(a+8)mol/L,��������Һ��C(OH-)��c(H��)=Kw=10-14. 10-(a+8) ��10-a=10-14.���a="3." ��������Һ��pH��3.(2) �������ʼ���ת����ϵ����֪��������֪A��CH4��B��C��C��CO��D��H2O��E��CO2��F��H2CO3��X��O2����B����Ԫ�������ڱ��е�λ�õڶ����ڢ�A�壬��д����������CO��Ӧ�Ļ�ѧ����ʽ��Fe2O3+3CO
OH-+H+������������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����c(H+)> c( SO42-) > c(NH4+) >c(OH��)�����ڳ����£�����NH3��H2O pH��a,C(H+)= 10-amol/L ,C(OH-)= 10(a-14)mol/L pH��b�����ᣬC(H+)=10-bmol/L ��a��b��14������C(H+)= C(OH-)�����ڼ�Ϊ����C(NH3��H2O)>C(HCl).��ǡ����ȫ��Ӧ�� V1��V2�Ĺ�ϵ��V1��V2����NH4Cl��HCl��PH��ȣ��������ǵ�PHΪX����ǰ��ˮ���������C(H+)=10-amol/L,������ˮ�������c(H��)Ϊ10-(a+8)mol/L, C(OH-)= c(H��)=10-(a+8)mol/L,��������Һ��C(OH-)��c(H��)=Kw=10-14. 10-(a+8) ��10-a=10-14.���a="3." ��������Һ��pH��3.(2) �������ʼ���ת����ϵ����֪��������֪A��CH4��B��C��C��CO��D��H2O��E��CO2��F��H2CO3��X��O2����B����Ԫ�������ڱ��е�λ�õڶ����ڢ�A�壬��д����������CO��Ӧ�Ļ�ѧ����ʽ��Fe2O3+3CO 2 Fe +3CO2����Ϊ�������ܶȻ�����KSpֻ���¶��йأ������ӵ�Ũ�ȴ�С�أ����Խ��ó�������0.1 mol��L��1��CaCl2��Һ�У���Ksp���䡣��ʱ����ɲ�����F������������Һ�е�Ũ��Ϊ2.8��10��9��0.1=2.8��10-8 mol/L.
2 Fe +3CO2����Ϊ�������ܶȻ�����KSpֻ���¶��йأ������ӵ�Ũ�ȴ�С�أ����Խ��ó�������0.1 mol��L��1��CaCl2��Һ�У���Ksp���䡣��ʱ����ɲ�����F������������Һ�е�Ũ��Ϊ2.8��10��9��0.1=2.8��10-8 mol/L.
���㣺����Ԫ�ء���������ƶϡ�����Ũ�ȵıȽϡ�Ӱ���ܶȻ����������ؼ�����Ũ�ȵļ����֪ʶ��


 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣���֪���ö��Ե缫���ij��X����Һ�������ķ�ӦΪ��
X+H2O  A�����ʣ�+B�����ʣ�+Y�������
A�����ʣ�+B�����ʣ�+Y�������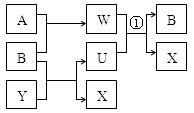
��1�������X��Ԫ�ؾ�Ϊ������Ԫ�أ���A��B��Ϊ���壬������ͬ��������� ��Ϊ1��1������֮����Է�����ͼ�ķ�Ӧ���仯�е�ˮ��ȥ����д���йط�Ӧ�����ӷ�Ӧ����ʽ����_______________________________��
��2����AΪ��ɫ���壬A������Y��Ũ��Һ��Ӧ����һ����A��Ħ��������ͬ�����塣
��д���õ������������ĵ缫��Ӧʽ��___________________��
��ijѧ�����һ��Ũ�ȵ�X��Һһ��ʱ�����������Һ�м���0.1molACO3�ָ������ǰ��Ũ�Ⱥ�pH��������CO2�ܽ⣩�����������ת�Ƶĵ�����Ϊ_______��
��3������X����Һ�м��������ϡ���ᣬ������ɫ�������Ҵ������µ�ת����ϵ��
��. A+Y(Ũ)��X+C(����)+H2O����. A+Y(ϡ)��X+D(����)+H2O����. M+O2��D(����)+H2O��
����֪�����M���������Ԫ��������Ϊ14:3�������M�ķ�����___________________��
���ڱ�״���£�27gA����һ��Ũ�ȵ�Y��Һʱ������2.8L���壬��װ�и��������Ͳ��������ˮ��ˮ���У���ͨ��_______L����B��ˮ����ǡ�ó�����Ͳ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2014��ȫ�˻��������ٰ죬�俪Ļʽ����һ�����ʢ�硣
��1����������˲��ֽ���Ԫ��������________��Ӧ���÷�Ӧ����________�����������ѧ�����仯��
��2����ͼ��ʾ����Ԫ������A��B��C��D��E���ֻ����ԲȦ���沿��ָ���ֻ����ﺬ��һ����ͬԪ�أ����ֻ����������ֶ�����Ԫ����ɣ�ÿ�ֻ������������Ԫ�ء�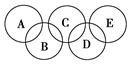
ͼ��A����������Ҫ�ɷ֣�B��E������������������Ϊ18��B���ȶ������н�ǿ�������ԣ���ϡ��Һ��ҽ���Ϲ㷺ʹ�õ���������E���ӽṹ�����ģ��Ϊ ��C�봿���ڸ����µķ�Ӧ�ǹ�ҵ�Ʋ�������Ҫ��Ӧ֮һ��D����������Ԫ�ص�ԭ�Ӹ�����Ϊ3��4����������֮��Ϊ3��2������������Ϣ�ش��������⣺
��C�봿���ڸ����µķ�Ӧ�ǹ�ҵ�Ʋ�������Ҫ��Ӧ֮һ��D����������Ԫ�ص�ԭ�Ӹ�����Ϊ3��4����������֮��Ϊ3��2������������Ϣ�ش��������⣺
��B��ˮ��Һ�������ԣ�����Ҫ�ĵ��뷽��ʽ�ɱ�ʾΪ________________��D�Ļ�ѧʽ��________��
��A��B��E�о����е�һ��Ԫ�ط���Ϊ________��
��C�봿�Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��Һ̬B��Һ̬E��Ӧ������һ�ֵ��ʺ�һ�ֳ���Һ�壬1 mol B�μӷ�Ӧ�ų�����Q kJ���䷴Ӧ���Ȼ�ѧ����ʽΪ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ����һЩ����Ԫ����ɵĵ��ʼ��仯����֮���ת����ϵͼ�����³�ѹ�£�D��F��Ϊ��ɫ��ζ�����壬B���������ɫҺ�壬A���ɵ���C��D��ȼ�����ɵĵ���ɫ���壻G��һ�ֺ��ɫ��������G��H��I��J�ж�����ͬһ��Ԫ�أ���Ӧ�����ɵIJ�����������ȥ������ش��������⣺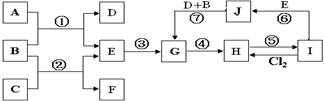
�ӷ�Ӧ�ڵ�ʵ��������Եó�����C���е�ijЩ���������У�д���㣩�� ��
��2��J��¶�ڿ����е������� ��
��3����--�ߵķ�Ӧ�����ڷ�������ԭ��Ӧ���� ��
��4����Ӧ�ٵĻ�ѧ����ʽΪ ��
��5����115g����(HCOOH)��ȫȼ�պ�IJ���ͨ�������Ĺ���A�У�����A������ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���п�ͼ��ʾ������ת����ϵ�У������ճ������г����Ľ������ҡ��������dz��������嵥�ʡ�����B������C�������������İ�������E��A��ǿ�D����ɫΪ��ɫ (���ַ�Ӧ��������Pˮ����ȥ)��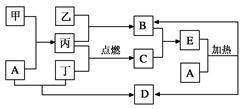
��ش���������
��1��д����ҵ�Ʊ�B�Ļ�ѧ����ʽ_____________________________________��
��2��д�������£�A�붡��Ӧ�����ӷ���ʽ________________________________��
��3����ͼװ��Ϊ���ſ������ռ������ʵ��װ��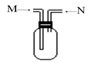
����M��ͨ������ʱ�����ռ��������� ��
����N��ͨ������ʱ�����ռ��������� �� �������ֱ�ţ�
�������� ������� �����嶡 ������B ������C
��4��E�����������ӵļ��鷽��Ϊ������ʵ�鲽�衢�����ۣ� ��
��5��A�����������ӵļ��鷽��Ϊ������ʵ�鲽�衢�����ۣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��G����ͼ��ʾ��ת����ϵ����������������ȥ��������A��GΪ���ʣ�D����ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬E��F������NaOH��Һ��Ӧ��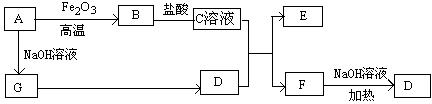
��ش��������⣺
��1��д��F�ĵ���ʽ��
��2����C��Һ��D��Ӧ�����ӷ���ʽΪ
��F��Һ��NaOH��Һ���ȷ�Ӧ�Ļ�ѧ����ʽΪ
��3�����������ӷ���ʽ����C��ҺΪ��������
��F��Һ������Ũ���ɴ�С��˳��Ϊ
��4����5.4gAͶ��200mL 2.0mol/Lij��Һ����G���ʲ������ҳ�ַ�Ӧ���н���ʣ�࣬�����Һ������ ������ţ�
A��HNO3��Һ B��H2SO4��Һ C��NaOH��Һ D��HCl��Һ
��5����1molN2��3molG�����������ݻ�Ϊ2L��ij�ܱ������н��з�Ӧ����֪�÷�ӦΪ���ȷ�Ӧ��ƽ��ʱ�����D�����ʵ���Ũ��Ϊa mol/L��
�������Ӧ����v(G)��1.2mol/(L��min)����v(D)�� mol/(L��min)
���������������������£�����ʼʱ����0.5molN2��1.5molG�ﵽƽ���D�����ʵ���Ũ�� ������ڡ�����С�ڡ����ڡ���a/2 mol/L��
�۸������µ�ƽ�ⳣ��Ϊ ���ú�a�Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ס��ҡ��������������ʣ���������Һ���У��ס��ҡ�����������ͬ��ij��Ԫ�أ�����֮���������ת����ϵ��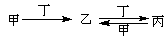 ����Ҫ��ش��������⣺
����Ҫ��ش��������⣺
��1������Ϊ̼������Ϊ________���ѧʽ����ͬ������Ϊ_________��
��2������Ϊ��������Ϊ_______����Ϊ_________��
��3������Ϊ��������Ϊ����������Һ��������ҵ����ӷ���ʽΪ_____________��
��4������Ϊ�Ȼ�����Һ����Ϊ����������Һ�����ҡ��������ӷ���ʽΪ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��A��B��CΪ��ѧ�����ĵ��ʣ�AΪ����ɫ���壻D��E��F��MΪ��ѧ�����������E�Ǵ��������Ҫ�ɷ֣��Ǻ�ɫ���壩��H��KΪ��ѧ�������Σ�M��һ�ֳ�������ɫҺ�塣�����ʼ��ת����ϵ����ͼ��ʾ��ijЩ��������ȥ����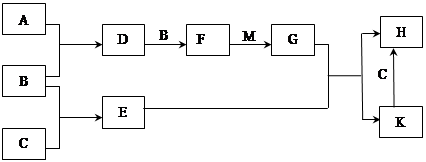
��ش�
��1������H�Ļ�ѧʽΪ ��
��2����F��M��������G�Ļ�ѧ��Ӧ��������Ϊ ��
��3��A��C�ڼ��������·�Ӧ�Ļ�ѧ����ʽΪ�� ��
��4��G��Ũ��Һ�ڼ����������ܸ�C��Ӧ���÷�Ӧ��G������ ������ţ���
A.������ B.��ԭ�� C.Ư���� D.����
��5�����Ƚ�D����ͨ��BaCl2��Һ�У���ͨ��NH3��ʵ������е�����Ϊ ��
��6��H��Һ�ڿ����г��ڷ��û���ֺ��ɫ���ǣ�����һ�����ӷ���ʽ��ʾ��仯��ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��J���ճ������г��������ֽ����������ֽ�����NaOH���ԭ��أ�A��������F�����������嵥�ʣ������������µ�ת����ϵ�����ֲ��P������ȥ����Ks5u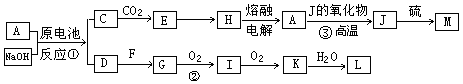
��ش��������⣺
��1��д����ԭ��ص��ܷ�Ӧ����ʽ_____________________��
��2��д���ڵĻ�ѧ����_________________��
��3������ʱpH=12��C��Һ�У����ʵ������������ʵ�������Ũ��֮��Ϊ ����д������ʽ��
��4��������J��������Ϊ�����������ÿ����1mol J�ų�Q kJ����������д��A��J��Ӧ���Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com