
(1)������������ʵ���Ũ�ȵĴ��������������Һ��Ϻ���Һ��________(����ԡ��������ԡ����ԡ�)����Һ��c(Na��)________c(CH3COO��)(�>������������<��)��
(2)pH��3�Ĵ����pH��11������������Һ�������Ϻ���Һ��________(����ԡ��������ԡ����ԡ�)����Һ��c(Na��)________c(CH3COO��)(�>������������<��)��
(3)���ʵ���Ũ����ͬ�Ĵ��������������Һ��Ϻ���Һ��CH3COO����Na��Ũ����ȣ����Ϻ���Һ��________(����ԡ��������ԡ����ԡ�)���������________����������Һ���(����ڡ��������ڡ���С�ڡ�)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ع㷺Ӧ���ڻ�϶�������ϵͳ���缫������Ni(OH)2��̼�ۡ���������Ϳ�����������Ƴɡ����ڵ��ʹ�ú�缫���϶Ի�����Σ����ij��ȤС��Ըõ�ص缫���Ͻ�����Դ�����о������ʵ���������£�
��֪����NiCl2������ˮ��Fe3����������Ni2������K (Ni(OH)
(Ni(OH) ): 5.0��10-16 ��K
): 5.0��10-16 ��K (NiC2O4): 4.0��10-10
(NiC2O4): 4.0��10-10
�ش��������⣺
��1�����ܺ�������������Ҫ�ɷ� �����������ƣ���
��2����NiO������Һ��pH�����������ijɷ�Ϊ____________________(�ѧʽ)����pH��ֽ�ⶨij��ҺpH�ľ�������� ��
��3��д������Na2C2O4��Һ��Ӧ�Ļ�ѧ����ʽ_____________________________��
��4��д������NaOH��Һ��������Ӧ�����ӷ���ʽ ���÷�Ӧ��ƽ�ⳣ��Ϊ ��
��5��������ɱ�������ò���֮һ ���ѧʽ����������Ni(OH)3 �����ϴ��Ni(OH)3 ������
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��100 ��ʱ��ˮ�������H+Ũ��Ϊ1.0��10-6 mol��L-1,���ʱKW=����������,c(OH-)=����������;25 ��ʱ��c(H+)=10-2 mol��L-1��������Һ��c(H+)=10-12 mol��L-1������������Һ�¶Ⱦ����ߵ�100 ��,H+Ũ�ȷֱ��Ƕ���?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)��������:��ʯī������������Һ̬�Ȼ��⡡�������������ڵ�����ء���ʳ�ξ��塡��������Һ�����Ҵ���������ᡡ��NH3��H2O,�����ܹ����������������,ǿ���������������,�����������������,�ǵ����������������
(2)H2S����ˮ�ĵ��뷽��ʽΪ��������������������
����H2S��Һ�м���CuSO4��Һʱ,����ƽ�������������ƶ�,c(H+)��������,c(S2-)����������
����H2S��Һ�м���NaOH����ʱ,����ƽ�������������ƶ�,c(H+)��������,c(S2-)����������
������H2S��Һ����������,c(H2S)����������
����Ҫ����H2S��Һ��c(S2-),��ü�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
| ʵ���� | HB���ʵ���Ũ��(mol/L) | KOH���ʵ���Ũ��(mol/L) | �����Һ��pH |
| �� | 0.2 | 0.2 | pH��a |
| �� | c1 | 0.2 | pH��7 |
| �� | 0.1 | 0.1 | pH>7 |
| �� | 0.1 | 0.1 | pH��9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�¶�(t ��)ʱ��ˮ�����ӻ�ΪKW��1.0��10��13mol2��
L��2������¶�(����ڡ�����С�ڡ����ڡ�)________25 �棬��������
____________________________________________________________��
�������¶���pH��11�Ŀ�������Һa L��pH��1��ϡ����b L���(���Ϻ���Һ�����С�仯���Բ���)����ͨ��������д���²�ͬ���ʱ������Һ������ȣ�
(1)�����û��ҺΪ���ԣ���a��b��________������Һ�и������ӵ�Ũ���ɴ�С����˳����____________________��
(2)�����û��Һ��pH��2����a��b��________������Һ�и������ӵ�Ũ���ɴ�С����˳����__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������أ�K2FeO4����ǿ�����ԣ���һ�ְ�ȫ�Ժܸߵ�ˮ��������
��1�������������Ԫ�صĻ��ϼ��� �������Խ���ˮ�е�������أ�KNO2��������ͬʱ���ɾ��������Ե�Fe(OH)3���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2��ijѧϰС���÷����ࣨ��Ҫ�ɷ�ΪFe3O3��FeO��CuO������Fe���Ʊ�������ص��������£�
�ٲ�����Ҫ��ͨ����н��У���ԭ���� ��
����ҺA�к��еĽ����������� ��
��25��ʱ��������ҺB�еĽ���������ȫ������Ӧ����c(H+)С�� ������֪��i. Ksp[Fe(OH)3]=2.7��10-39��Ksp[Fe(OH)2] =4.0��10-17��Ksp[Cu(OH)2] =1.6��10-20��ii. ��Һ������Ũ��С��10-5 mol��L-1ʱ������Ϊ������ȫ����
�ܸ�С�龭���ʵ�飬�õ���ͼ��������ǵ�ʵ��Ŀ���� ��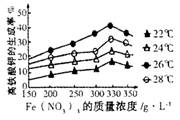
��3��������ػ������õ�ⷨ��ȡ����������˿��Ϊ���������Һʹ������������Һ����������ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��25��ʱ������HA���MOH��������.
��1����0.01mol/L��ǿ��HA��0.01mol/Lǿ��MOH��ϣ���������Һ�� ������ԡ��������ԡ����ԡ�����ͬ���÷�Ӧ�����ӷ���ʽΪ
��2����PH=3��ǿ��HA��PH=11������MOH��ϣ���������Һ�� �������ǣ�
��3����0.01mol/L��ǿ��HA��0.01mol/L����MOH��ϣ���������Һ�� ��������һ��������ӷ���ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ba�����ȣ�Sr�����仯�����ڹ�ҵ�����Ź㷺��Ӧ�ã������ڵؿ��г��������ε���ʽ���ڣ�BaSO4��SrSO4�����������Ρ���ҵ����ȡ������ʱ���Ƚ�BaSO4��SrSO4ת�������������Ρ���֪��
SrSO4��s�� Sr2����aq����SO42-��aq����Ksp��2.5��10��7
Sr2����aq����SO42-��aq����Ksp��2.5��10��7
SrCO3��s�� Sr2����aq����CO32-��aq����Ksp��2.5��10��9
Sr2����aq����CO32-��aq����Ksp��2.5��10��9
��1����SrSO4ת����SrCO3�����ӷ���ʽΪ________________________���÷�Ӧ��ƽ�ⳣ������ʽΪ____________���÷�Ӧ�ܷ�����ԭ����_________________________________________________________________________________________________________________________________________________�����ó����ܽ�ƽ����й����۽��ͣ�
��2������������Ӧ��ʵ��֤������CO32-��Ũ�Ȼ��¶ȶ����������SrSO4��ת���ʡ��ж���������������£�ƽ�ⳣ��K�ı仯��������������С�����䡱����
�������¶ȣ�ƽ�ⳣ��K��________��
������CO32-��Ũ�ȣ�ƽ�ⳣ��K��________��
��3����֪��SrSO4��SrCO3�����е��ܽ�����BaSO4��BaCO3���ƣ����ʵ��֤������������SrSO4�Ƿ���ȫת����SrCO3��
ʵ�����õ��Լ�Ϊ________��ʵ����������Ӧ����Ϊ_____________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com