
����ʯ��������õ�����[KAl(SO4)2��12H2O]���������Ʊ�Al��K2SO4����H2SO4�Ĺ��չ���������ʾ��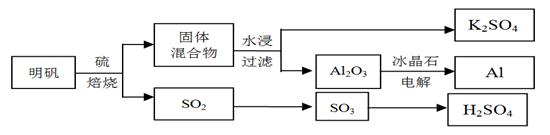
���������Ļ�ѧ����ʽΪ��4KAl(SO4)2��12H2O + 3S = 2K2SO4 + 2Al2O3 + 9SO2 + 48H2O
��ش��������⣺
��1���ڱ��������ķ�Ӧ�У���ԭ���� ��
��2����ˮ�������Һ�еõ�K2SO4����ķ����� ��
��3��Al2O3��һ�������¿��Ƶ�AlN���侧��ṹ��ͼ��ʾ���þ�����Al����λ���� ��
��4����Al��NiO(OH)Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO(OH)ת��ΪNi(OH)2���õ�ط�Ӧ�Ļ�ѧ����ʽ�� ��
��5�����ղ�����SO2�����������ᡣ��֪25�桢101kPaʱ��
2SO2��g��+ O2��g�� 2SO3��g�� ��H1 = ��197 kJ /mol��
2SO3��g�� ��H1 = ��197 kJ /mol��
H2O��g�� H2O��l�� ��H2 = ��44 kJ/mol��
H2O��l�� ��H2 = ��44 kJ/mol��
2SO2��g��+ O2��g��+ 2H2O��g��=2H2SO4��aq�� ��H3 = ��545 kJ/mol��
��SO3��g����H2O��l����Ӧ���Ȼ�ѧ����ʽ�� ��
����948 t������M =" 474" g/mol������SO2��������Ϊ96%���ɲ�����������Ϊ98%������ t��
��1��S ��2�֣�
��2�������ᾧ��2�֣�
��3��4 ��2�֣�
��4��Al+3NiO(OH)+H2O==NaAlO2+3Ni(OH)2 ��3�֣�
��5��SO3(g)+H2O(l)==H2SO4(aq), ��H="-130" KJ/mol ��3�֣� ��432t ��3�֣�
�����������������������ԭ��Ӧ�����ʵķ�����ᴿ�����ʵĽṹ��֪ʶ���ںϵ����չ����У���������ԭ���ԭ�����Ȼ�ѧ����ʽ����д���Ѷ����С�
���㣺������ԭ��Ӧ��ԭ���ԭ�����Ȼ�ѧ����ʽ����д��


 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о���ѧϰС���Na2O2���й�����̽�����£�����з�̪��ˮ��Ͷ��һ������Na2O2���۲쵽���д������ݲ�������Һ��죬��һ�����Һ�ֱ�Ϊ��ɫ��
������ʵ������Һ��죬��һ�������ɫ��ԭ�ס�����ͬѧ����˲�ͬ�Ľ��ͣ�
��ͬѧ��Ϊ��Na2O2��ˮ��Ӧ�ų������������������ԣ�����̪������ʹ��Һ��ɫ��
��ͬѧ����Ϊ��Na2O2��ˮ��Ӧʱ������H2O2��H2O2��ǿ������ʹ��̪��ɫ��
(1)��ͬѧ���������ʵ����֤���Լ��IJ�������ȷ�ģ��ڵ��з�̪������������Һ�еμ�3%��H2O2��Һ����
������ͬѧ�IJ�����ȷ���ɹ۲쵽�������� ��
�ڼ�ͬѧ�����ͬѧ��������֤ʵ�������Ӧ����ʵ����ܸ�ֱ��֤��Na2O2��ˮ��Ӧ������H2O2����ͬѧ���Ҫ���ӵ�ʵ����ʲô��
(2)����Ƽ�ʵ��֤����ͬѧ�Ľ����Ƿ���ȷ(��������װ��ͼ��˵����Ҫ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������(��Ҫ�ɷ���Al2O3����SiO2��Fe2O3��MgO������)����ȡ�����������ֹ����������£�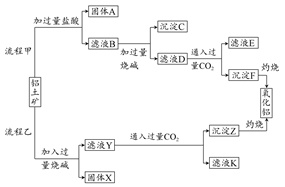
��ش��������⣺
(1)���̼������������Al3�������ӷ���ʽΪ ��
(2)�����Ҽ����ռ������SiO32-�����ӷ���ʽΪ ��
(3)��֤��ҺB��Fe3������ȡ������Һ������ (���Լ�����)��
(4)��ҺE��K�����ʵ���Ҫ�ɷ��� (�ѧʽ)��д�������ʵ�һ����;�� ��
(5)����298 Kʱ��Mg(OH)2���ܶȻ�����Ksp��5.6��10��12��ȡ��������ҺB������һ�������ռ����ﵽ�����ܽ�ƽ�⣬���pH��13������¶��²�������Һ�е�c(Mg2��)�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ȡA��B�������ʵ���Ũ����ȵ�NaOH��Һ�������Ϊ50 mL���ֱ�������ͨ��һ������CO2���ٷֱ�ϡ��Ϊ100 mL��
(1)��NaOH��Һ��ͨ��һ������CO2����Һ�е����ʵ���ɿ����ǣ�
�� ���� ���� ���� ��
(2)��ϡ�ͺ����Һ�зֱ���μ���0.1 mol��L��1�����ᣬ������CO2�����(��״��)����������������ϵ��ͼ��ʾ��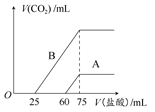
�ٷֱ�����������������Һ�е������� ��ԭNaOH��Һ�����ʵ���Ũ���� ��
��A���߱�����ͨ��CO2����Һ�е������� �������ᷴӦ����CO2���������� mL(��״��)��
��B���߱�����ԭNaOH��Һͨ��CO2���������ʵĻ�ѧʽΪ �������ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ������£�
(ע��Al(OH)3��Cu(OH)2��ʼ�ֽ���¶ȷֱ�Ϊ450 ���80 �档)
(1)��⾫����ʱ��������ӦʽΪ________������A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���������ɫ�Ļ�ѧ��Ӧ����ʽΪ________________��
(2)��������B�����Ϊ__________�������ɹ���B�Ĺ����У������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ____________��
(3)������չ�����һ����Ӧ�Ļ�ѧ����ʽ��
CuO��____Al2O3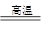 ____CuAlO2��________����
____CuAlO2��________����
(4)����ͭ�Ͻ���ͭ����������Ϊ63.5%��������5.0 kg�����е�ͭ����ȫת��Ϊ________ mol CuAlO2��������Ҫ1.0 mol��L��1��Al2(SO4)3��Һ________ L��
(5)CuSO4��ҺҲ�������Ʊ������������������________�����ˡ�ϴ�Ӻ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������Ļ�����Ӧ�ù㷺����FeCl3������������ӡˢ��·ͭ�帯ʴ��������ֹѪ���ȡ�
��1��д��FeCl3��Һ��ʴӡˢ��·ͭ������ӷ���ʽ��
________________________________________________________________��
��2��������1���еķ�Ӧ��Ƴ�ԭ��أ��뻭��ԭ��ص�װ��ͼ�����������������д���缫��Ӧʽ��
������Ӧ�� _________________________________________��
������Ӧ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ����ͭ������ˮ�����ȵ�210������������Cu(NO3)2 ��3H2O����Ļ�ѧ�����нϴ����, Cu(NO3)2 ��3H2O������ȵ�170��ֽ⡣��֪:���������ķе�Ϊ77 �档
��1����������Cu(NO3)2 ��Һ�ò�����ˮ����ͭ��ԭ����_____________��
��2����ͭƬ���˵�N2 O 4������������Һ�п��Ƶ���ˮ����ͭ��ͬʱ����NO,д����Ӧ�Ļ�ѧ����ʽ_____________���������Ҵ��з������ˮ����ͭ��ʵ�������_____________��
��3��Ϊ̽��Cu(NO3)2 ��3H2O���ȷֽ�IJ��ij̽��С��������ͼװ�ý���ʵ�顣(ͼ�мгֺͼ���װ��ȥ)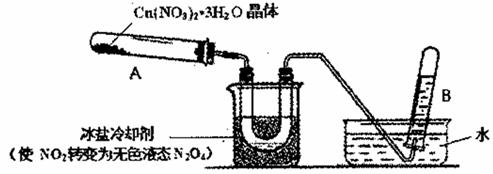
���Թ�A�м�����ϸ��Cu(NO3)2 ��3H2O�岢���ȣ��۲쵽�Թ�A���к���ɫ�����ɣ����ղ�����ɫ��ĩ��U������Һ�����ɣ����Թ�B���ռ�����ɫ���塣
�ٵ����ܿڲ���������ð��ʱ����Ӧֹͣ�����װ�õIJ���������______��
���Թ�B���ռ���������һ������______��
��4��п��Cu(NO3)2��Һ�ܷ�����Ӧ����һ֧�Թ���ע��1 mol��L-1��Cu(NO3)2��Һ���ٷ���һ��пƬ���۲쵽�ڷ�Ӧ�����д�����ɫ����ð����ͬʱпƬ��������ɫ���塣��С���������ijɷ֣�����Ƶ�ʵ�鲽�裬����д�±���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������һ����Ҫ�Ļ��������м����,��Ҫ�ɷ���Fe3O4��Fe2O3��FeO�Ͷ�������ȡ��������������������Ʊ���Ч��ˮ���ۺ�������������ͼ: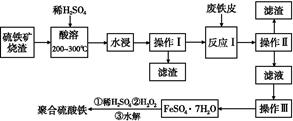
(1)ʵ����ʵ�֡����������õIJ����������������������������ձ�����������ϵ�в�����������Ϊ�����������������������˺�ϴ�ӡ�
(2)�����ܡ�������Fe3O4�ܽ�Ļ�ѧ��Ӧ����ʽΪ ��
(3)�����������ڡ����ܡ�ǰҪ�������ҪĿ���� ��
(4)ʵ���Ҽ��顰��Ӧ���Ѿ���ȫ���Լ�����������,������ ��
(5)��������H2O2��Ŀ��������Fe2+,д��H2O2����Fe2+ΪFe3+�����ӷ���ʽ: ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ȡ��������Ŀ�������Ҫ�ɷ�ΪFe2O3��Fe3O4��FeO��Al2O3��SiO2�ȣ��Ʊ��ߴ�����������-Fe2O3���Ĺ����������£�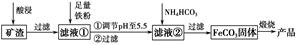
��1���������������Fe3O4������Ӧ�����ӷ���ʽΪ______________________________��Ϊ��ߡ����������Ԫ�صĽ����ʣ����˲��ú��ʵ�Һ�̱Ⱥ�ѭ����ȡ�⣬���˵���������____________________��____________________�����ξ�������
��2������pH��5.5��Ŀ����______________________________________��
��3����Һ���м���NH4HCO3ʱ��Ҫ���Ʒ�Ӧ�¶Ȳ��ܹ��ߣ�ԭ����__________________________________________________������һ�㼴�ɣ�
��4���ڿ���������FeCO3�Ʊ��ߴ��������Ļ�ѧ����ʽΪ_______________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com