
��֪��2KMnO4��16HCl===2KCl��2MnCl2��5Cl2����8H2O��K2Cr2O7��14HCl===2KCl��2CrCl3��3Cl2����7H2O��MnO2��4HCl MnCl2��Cl2����2H2O������KMnO4��һ��Ũ�ȵ����ἴ�ɷ�Ӧ��K2Cr2O7��ͽ�Ũ����(>6 mol��L��1)��Ӧ��MnO2���Ũ����(>8 mol��L��1)��Ӧ������������Ϣ�����н����в���ȷ���� (����)��
MnCl2��Cl2����2H2O������KMnO4��һ��Ũ�ȵ����ἴ�ɷ�Ӧ��K2Cr2O7��ͽ�Ũ����(>6 mol��L��1)��Ӧ��MnO2���Ũ����(>8 mol��L��1)��Ӧ������������Ϣ�����н����в���ȷ���� (����)��
A��������Ӧ������������ԭ��Ӧ
B������1 mol Cl2ʱ��������Ӧ��ת�Ƶĵ��������
C������Ũ��Խ��Cl���Ļ�ԭ��Խǿ
D�������ԣ�KMnO4>K2Cr2O7>Cl2>MnO2
 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������˵������ȷ���� (����)��
A�������ʵ�����CO2��NH3�������ķ�������ΪNA
B����״���£�33.6 L SO3�к��е���ԭ������4.5NA
C�������£�34.5 g NO2��N2O4�Ļ�����к���1.5NA����ԭ��
D����⾫��ͭʱ��ÿת��2NA�����ӣ������ܽ�64 gͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ͼ�Ӳ�ʺϽ��к�̼����(WC)��������(Co)������������������ ��ⷨ�ɻ���WC��Co���������̼�ͼ���£�
��ⷨ�ɻ���WC��Co���������̼�ͼ���£�

 (1)���ʱ�Ͼɵ������������������������HCl��ҺΪ���Һ��������Ҫ�ĵ缫��ӦʽΪ____________________________________________________��
(1)���ʱ�Ͼɵ������������������������HCl��ҺΪ���Һ��������Ҫ�ĵ缫��ӦʽΪ____________________________________________________��
(2)�������������˱�����Ҫ�ɷ���____________�����յ�ϴ��Һ���� ˮ���Ƶ��Һ��Ŀ���ǻ����������е�____________��
ˮ���Ƶ��Һ��Ŀ���ǻ����������е�____________��
(3)��Һ�����Ҫ�ɷ���____________��ϴ��CoC2O4����ֶ����ղ�Ʒ���Ȳ�������Ӱ�죬������ʱ����ɻ�����Ⱦ��ԭ����_______________________ _________________________________________________��
(4)��Co2O3��ԭ��Co�۵Ļ�ѧ��Ӧ����ʽΪ________________________ _________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ӷ���ʽ��д��ȷ���� (����) ��
��
A��NaClO��Һ��FeCl2��Һ��ϣ�6Fe2����3ClO����3H2O===2Fe(OH)3����
3Cl����4Fe3��
B����ʳ���������е�̼��ƣ�CaCO3��2H��===Ca2����CO2����H2O
C��FeCl2������Һ���ڿ����б��ʣ�2Fe2����4H����O2===2Fe3����2H2O
D�����MgCl2ˮ��Һ�����ӷ���ʽ��2Cl����2H2O H2����Cl2����2OH��
H2����Cl2����2OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�����ų�����ˮ�к��д�����Fe2����Zn2����Hg2�����ֽ������ӡ�������ij��ѧ�о���ѧϰС���ͬѧ��Ƴ�ȥ��ˮ�еĽ������ӣ��������̷���𩷯(ZnSO4��7H2O)���ķ�����
��ҩƷ����NaOH��Һ��������Һ����������ϡ���ᡢ����
��ʵ�鷽����
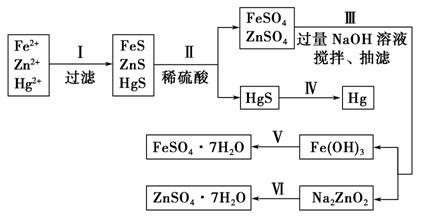
������̽����
(1)�������������Ӧ�����ӷ���ʽΪ_________________________________��
(2)������г��˵�Ŀ����____________���ò������Fe(OH)3�ķ�Ӧ�����ӷ���ʽΪ_________________ _____________________________________��
_____________________________________��
(3)������еõ�����п��Һ�����ӷ���ʽΪ___________________________ _____________________________________________________________��
(4)��ʵ�ֲ�������������Լ���________��________�����漰����Ҫ��������Ϊ__________________________________________________________��
(5)��������õķ�����________���ò����Ƿ�Ի�����Ӱ�죿________(��ǡ��� ��)������Ӱ�죬�������һ����ɫ����������ʵ�ֲ�����ķ�Ӧ��_____________________________________________________________��
��)������Ӱ�죬�������һ����ɫ����������ʵ�ֲ�����ķ�Ӧ��_____________________________________________________________��
(6)���о�С���ͬѧ��ǿ����Һ�У��ô���������Fe(OH)3��Ӧ����˸�Ч��ˮ��Na2FeO4���÷�Ӧ�����ӷ���ʽΪ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)����Ѫ�쵰�����к���Fe2����������ЩFe2��ʹѪ�쵰���Ӿ����������ܡ���������(NaNO2)�ɽ�����Ѫ�쵰���е�Fe2��ת��ΪFe3�������ɸ���Ѫ�쵰��ɥʧ�������Ľ����������Ӧ������Fe2������________��Ӧ��˵���������ƾ���________�ԣ���ʳ���������ж����ɷ�ά����C���⣬˵��ά����C����________�ԡ�
 (2)ϡ���������ǽ����Ȼ�ԭ����ȡ��һϡ����������Ҫԭ�ϡ������ϳ�CeF3�Ļ�ѧ����ʽΪ6CeO2��18NH4F===6CeF3��16NH3����12H
(2)ϡ���������ǽ����Ȼ�ԭ����ȡ��һϡ����������Ҫԭ�ϡ������ϳ�CeF3�Ļ�ѧ����ʽΪ6CeO2��18NH4F===6CeF3��16NH3����12H 2O��N2�����÷�Ӧ����������________����ԭ����________����Ӧ�б������ĵ�ԭ����δ�������ĵ�ԭ�����ʵ���֮��Ϊ________��
2O��N2�����÷�Ӧ����������________����ԭ����________����Ӧ�б������ĵ�ԭ����δ�������ĵ�ԭ�����ʵ���֮��Ϊ________��
(3)��������﮵ĺ��⻯������Ϊ��Դ�ܵ��㷺��ע��������LiNH2��LiH���Ǿ���DZ��Ӧ�ü�ֵ��������ϡ���LiNH2��LiH��һ��������ϣ��ڴ��������£�������ȫ����������ʽ�ų�ͬʱ����Li3N����Ӧ�Ļ�ѧ����ʽΪ_________________________________________________________________��
��Ӧ��ÿ����0.1 mol H2��ת�Ƶ�����Ϊ________NA��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������˳����ȷ���ǣ� ��
A�����ȶ��ԣ�CH4>SiH4>HF B.ԭ�Ӱ뾶��Na>Mg>O
C�����ԣ�HClO4>H2SO4>H3PO4 D.�ǽ����ԣ�F>Cl>Br
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���� �� ��
A����ⱥ��ʳ��ˮʱ�������ĵ缫��ӦʽΪ��2Cl�� ��2e��=��Cl2 ��
B������ȼ�ϵ�صĸ�����Ӧʽ��O2 + 2H2O + 4e- == 4OH��
C����ͭ����ʱ�����Դ�����������Ǵ�ͭ
D�����������绯��ʴ��������Ӧʽ��Fe��2e�� == Fe2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ������Cu��Fe2O3�Ļ�����м���300 mL 1 mol��L��1�����ᣬǡ��ʹ�������ȫ�ܽ⣬������Һ�в���Fe3�������ù�����CO�ڸ����»�ԭ��ͬ������ԭ����������ٵ�����Ϊ (����)��
A��6.4 g B��4.8 g C��2.4 g D��1.6 g
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com