
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ������84����Һ��ͨ��ϡ��100��(���֮��)��ʹ�á���ش��������⣺
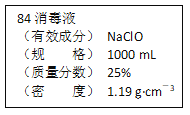
(1)����84����Һ�������ʵ���Ũ��ԼΪ_____mol��L��1��
(2)ȡ����������ĸ�����Һʱ�������������л�����ȡ����Ķ��ٶ��仯����________(����ĸ)��
A����Һ��NaClO�����ʵ��� B����Һ��Ũ��
C����Һ��NaClO��Ħ������ D����Һ���ܶ�
(3)��ͬѧ���ĸ���84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ25%������Һ���ش��������⡣
����ͼ��ʾ�������У���Щ�Dz���Ҫ������������Һ����Ҫ��������_______

����Ҫ����NaClO���������Ϊ_______ g
(4)��84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ��������200 mL 2.3 mol��L��1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H�������ʵ���Ũ��Ϊ________mol��L��1��
������Ũ��������Ϊ________ mL��
���������Ƶ�ϡ����Ũ��ƫС�������п��ܵ�ԭ���������ȷ����_______��
A������ǰ������ƿ������������ˮ B����ȡŨ����ʱ������Һ��İ�Һ��
C��δ��ȴ������ת��������ƿ���� D������ʱ��������Һ�İ�Һ��
���𰸡�4.0 A ������,��ͷ�ι� 148.8 4.6 25.0 D
��������
(1)����84����Һ�������ʵ���Ũ��ԼΪ![]() mol��L��1��
mol��L��1��
(2)A����Һ��NaClO�����ʵ�������Һ����йأ���Һ���Խ��NaClO�����ʵ���Խ�ࣻ
B����Һ�Ǿ�һ�ȶ��ģ���Ũ�Ⱥ�����ء�
C��NaClO��Ħ��������74.5g/mol������Һ����ء�
D����Һ�Ǿ�һ�ȶ��ģ���Һ���ܶȺ�����ء�
��ѡA��
(3)����480 mL��NaClO��������Ϊ25%������Һ������ʵ����û��480mL������ƿ������Ҫ��500mL������ƿ����500mL����Һ����Ҫ�ȼ������ҪNaClO��������Ȼ������ƽ�Ƴ�NaClO���壬�����ձ��У���������ˮ���ò����������ܽ��ת�Ƶ�����ƿ�У�������ˮϴ�����õ��ձ��Ͳ�����2~3�Σ���ϴ��ҺҲת�Ƶ�����ƿ�У�Ȼ��ֱ��������ƿ�м�����ˮ����̶���1~2cm�ĵط������ý�ͷ�ιܵμ�����ˮ���̶��ߣ�������µߵ�ҡ�ȡ�
����Ҫ���������ձ�����������������ƽ��500mL����ƿ����ͷ�ιܣ����˸������ձ���������ƽ������ƿ�⣬����Ҫ�������ͽ�ͷ�ιܡ�
�ڼ������NaClO�����������500mL��1.19g��cm-3��25%=148.8g��
(4)��2.3 mol��L��1��ϡ�����У�H�������ʵ���Ũ��Ϊ2.3 mol��L��1��2=4.6mol��L��1��
��Ũ��������ʵ���Ũ��Ϊ![]() mol/L������ϡ��ǰ�����ʵ����ʵ������䣬�ɼ�������200 mL 2.3 mol��L��1��ϡ������Ҫ��Ũ��������Ϊ
mol/L������ϡ��ǰ�����ʵ����ʵ������䣬�ɼ�������200 mL 2.3 mol��L��1��ϡ������Ҫ��Ũ��������Ϊ![]() ����25.0 mL��
����25.0 mL��
��A������ǰ������ƿ������������ˮ����Ӱ�����ʵ����ʵ�����Ҳ��Ӱ����Һ����������Զ�������Һ��Ũ����Ӱ�죻
B����ȡŨ����ʱ������Һ��İ�Һ�棬�ᵼ����ȡ��Ũ����ƫ�࣬���Ƶ�ϡ�����Ũ��ƫ�ߣ�
C��δ��ȴ������ת��������ƿ���ݻᵼ����Һ���ƫС�����Ƶ�ϡ�����Ũ��ƫ�ߣ�
D������ʱ��������Һ�İ�Һ��ᵼ����Һ���ƫ�����Ƶ�ϡ�����Ũ��ƫ�͡�
��ѡD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������������ȷ����( )
A. ���³�ѹ�£�11.2L����������������ԭ��������NA
B. 0.5molH2O�����ĵ�����Ϊ9NA
C. 8.0gCu2S��CuO�Ļ�����к���ͭԭ����Ϊ0.1NA
D. 300mL2mol��L-1������Һ������������Ϊ0.6NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��������Ϊ16��7��6����������SO2��CO��NO�����Ӹ���֮��Ϊ_____________����ԭ�Ӹ���֮��Ϊ____________��
��2���ڱ�״���£�6.8g PH3���״����_______L CH4������ͬ��Ŀ��Hԭ�ӡ�
��3��ij���������ﻯѧʽΪRO2���ڱ�״���£�1.28 g������������Ϊ448 mL������������Ħ������Ϊ________��R�����ԭ������Ϊ________��
��4��273 K��1.01��105 Paʱ��̬����X2���ܶ�Ϊ1.25 g��L-1����X�����ԭ������Ϊ________
��5����ͬ�¶Ⱥ�ѹǿ�����£�һ���������̬�⻯��H2R�������ǵ����NH3��2������R�����ԭ������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ��������������к���Cu��Au(��)��PbSO4�����ʣ�ʪ����������������ۺ����õĹ���������ͼ��ʾ��
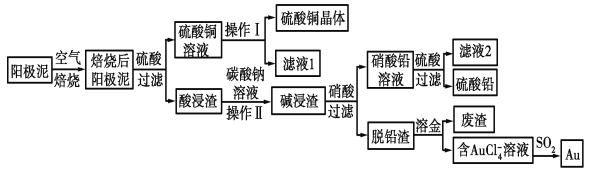
��1����⾫����ͭ����Ǧ�Ĵ�ͭʱ�����ҺӦ����________��Һ�����Һ�����ʱ�����ĵ缫��ӦʽΪ___________________________��Cu��2e��===Cu2����
��2����ɲ���������Ҫ�����У�__________________�����ˣ�ϴ�ӣ����
��3��д����SO2��ԭAuCl4-�����ӷ�Ӧ����ʽ____________________________��
��4��Ϊ�˼��ٷ�Һ�ŷš��������������Դ����ҵ�Ͻ���Һ1��������ͭ��Һ����ѭ����������ָ������ͼ����һ�����Ƶ�����________________________��
��5�������ӷ���ʽ��ʾ����̼������Һ�����ã�___________________________��[��֪298 Kʱ��Ksp(PbCO3)��1.46��10��13��Ksp(PbSO4)��1.82��10��8]������Һ��c(SO42-)=0.2mol/Lʱ��c(CO32-)=_______________mol/L�����������2λ��Ч���֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������Ƽ����Ҫ��Ӧԭ���ǣ�NH3+CO2+H2O+NaCl=NaHCO3��+NH4Cl����ʵ���Ҹ��ݴ�ԭ���Ʊ�������Na2CO3����Ҫʵ���������ȡNH3��CO2������NaHCO3������NaHCO3����ȡNa2CO3 �ĸ����衣����ʵ��ѡ�õ���Ҫ��������Ҫ���費��ȷ����
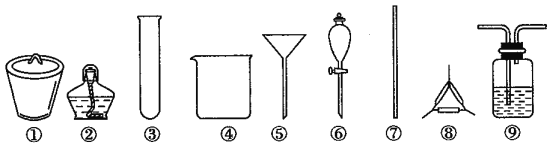
A. ��ȡ��������ѡ�âڢ�
B. ���� NaHCO3����ѡ�âܢݢ�
C. ��ȡ Na2CO3����ѡ�â٢ڢߢ�
D. ��ȡ NaHCO3ʱ��Ӧ���ڢ���ͨ��CO2���ټ��백ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����V mL 0.1 mol/L��ˮ�еμӵ����ʵ���Ũ�ȵ�ϡH2SO4����û����Һ���¶Ⱥ�pOH [pOH����lgc(OH��)]���ż���ϡ���������ı仯��ͼ��ʾ��ʵ��Ϊ�¶ȱ仯������ΪpOH�仯��������˵������ȷ����

A. V ��40
B. b��ʱ��Һ��pOH > pH
C. a��b��c������ˮ�����c��OH-�����μ�С
D. a��b��d�����ӦNH3��H2O�ĵ��볣����K(b)>K(d)>K(a)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������İ���ͭ����([Cu(NH3)4]SO4��H2O)������ɱ�����ýȾ�����ڼ��Զ�ͭ��Ҳ���������Һ����Ҫ�ɷ֣��ڹ�ҵ����;�㷺�������¸������ڿ����в��ȶ�������ʱ�����ֽ⡣ij��ѧ��ȤС����Cu�ۡ�3mol/L�����ᡢŨ��ˮ��10% NaOH��Һ��95%���Ҵ���Һ��0.500 mol/Lϡ���ᡢ0.500 mol/L��NaOH��Һ���ϳ������İ���ͭ���岢�ⶨ�䴿�ȡ�
I��CuSO4��Һ���Ʊ�
�ٳ�ȡ4gͭ�ۣ���A����������10���Ӳ����Ͻ��裬������ȴ��
�����������м���30mL 3mol/L�����ᣬ��A�й��������������У����Ȳ����Ͻ��衣
�۳��ȹ��˵���ɫ��Һ��
(1)A����������Ϊ________________________________��
(2)ijͬѧ��ʵ������1.5g��ͭ��ʣ�࣬��ͬѧ���Ƶõ�CuSO4��Һ������һ�������м���Ũ�����о�Ĥ���֣���ȴ�����ľ����к��а�ɫ��ĩ���Խ�����ԭ��__________________________________________��
II��������Ʊ�
�������Ʊ���CuSO4��Һ����ͼ��ʾ���в���
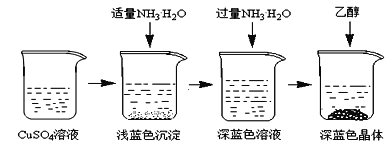
(3)��֪dz��ɫ�����ijɷ�ΪCu2(OH)2SO4����д�����ɴ˳��������ӷ�Ӧ����ʽ___________________��
(4)��������ʱ���ü����Ҵ��ķ�����������Ũ���ᾧ��ԭ����________________________��
III���������IJⶨ
��ȷ��ȡwg������������ˮ�ܽ���ע����ͼ��ʾ������ƿ����Ȼ����μ�������10%NaOH��Һ��ͨ��ˮ����������ƷҺ�еİ�ȫ����������������ˮ��ϴ�����ڱڣ���V1mL0.5mol/L���������Һ��ȫ���ա�ȡ�½���ƿ����0.5mol/L NaOH����Һ�ζ���ʣ��HCl(ѡ�ü�����ָʾ��)�����յ�ʱ����V2mLNaOH��Һ��
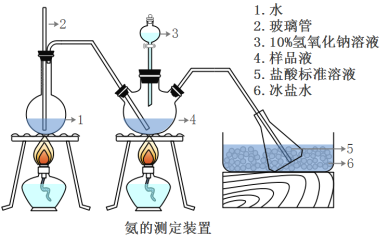
(5)Aװ���г������ܵ�����_________________����Ʒ�а������������ı���ʽ_______��
(6)����ʵ���������ʹ�������ⶨ���ƫ�ߵ�ԭ����____________________��
A���ζ�ʱδ��NaOH����Һ��ϴ�ζ��ܡ�
B������ʱ���ζ�ǰƽ�ӣ��ζ����ӡ�
C���ζ�������ѡ�÷�̪��ָʾ����
D��ȡ�½���ƿǰ��δ������ˮ��ϴ�������ƿ�еĵ�����ڡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������̼�Ļ�����������������Ӧ�ù㷺��
��1�����Ȱ���NH2Cl���ĵ���ʽΪ_______________��
��NH2Cl��ˮ��Ӧ����ǿ�����Ե����ʣ�������Ч�������������÷�Ӧ�Ļ�ѧ����ʽΪ__________________��
��2���ý�̿��ԭNO�ķ�ӦΪ��2NO(g)+C(s)![]() N2(g)+CO2(g)�����ݻ���Ϊ1L�ļס��ҡ����������ݺ��£���Ӧ�¶ȷֱ�Ϊ400�桢400�桢T�棩�����зֱ���������Ľ�̿��һ������NO����ø�������n��NO���淴Ӧʱ��t�ı仯������±���ʾ��
N2(g)+CO2(g)�����ݻ���Ϊ1L�ļס��ҡ����������ݺ��£���Ӧ�¶ȷֱ�Ϊ400�桢400�桢T�棩�����зֱ���������Ľ�̿��һ������NO����ø�������n��NO���淴Ӧʱ��t�ı仯������±���ʾ��
t/min | 0 | 40 | 80 | 120 | 160 |
n��NO������������/mol | 2.00 | 1.50 | 1.10 | 0.80 | 0.80 |
n��NO������������/mol | 1.00 | 0.80 | 0.65 | 0.53 | 0.45 |
n��NO������������/mol | 2.00 | 1.45 | 1.00 | 1.00 | 1.00 |
�ٸ÷�ӦΪ_______________������ȡ������ȡ�����Ӧ��
����������200min�ﵽƽ��״̬����0��200min����NO��Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(NO)=___________��
��3���ý�̿��ԭNO2�ķ�ӦΪ��2NO2(g)+2C(s)![]() N2(g)+2CO2(g)���ں��������£�1mol NO2������C�����÷�Ӧ�����ƽ��ʱNO2��CO2�����ʵ���Ũ����ƽ����ѹ�Ĺ�ϵ����ͼ��ʾ��
N2(g)+2CO2(g)���ں��������£�1mol NO2������C�����÷�Ӧ�����ƽ��ʱNO2��CO2�����ʵ���Ũ����ƽ����ѹ�Ĺ�ϵ����ͼ��ʾ��

��A��B�����Ũ��ƽ�ⳣ����ϵ��Kc��A��_____Kc��B���������������=������
��A��B��C������NO2��ת������ߵ���__________���A����B����C�����㡣
�ۼ���C��ʱ�÷�Ӧ��ѹǿƽ�ⳣ��Kp��C��=________��Kp����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�����������
��4�������о����֣��ø�Ĥ��ⷨ���Դ�����Ũ����ȩ��ˮ��ԭ����ʹ�ö��Ե缫��⣬��ȩ�ֱ�����������ת��Ϊ�Ҵ������ᣬ�ܷ�ӦΪ��2CH3CHO+H2O![]() CH3CH2OH+CH3COOH��ʵ�����У���һ��Ũ�ȵ���ȩ-Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ���������̣���װ��ʾ��ͼ����ͼ��ʾ��
CH3CH2OH+CH3COOH��ʵ�����У���һ��Ũ�ȵ���ȩ-Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ���������̣���װ��ʾ��ͼ����ͼ��ʾ��

����д���������У������ĵ缫��Ӧʽ��_________________________��
����ʵ�ʴ��������У�����·��I=50Aʱ��10min������ȩ8.8g�������Ч��Ϊ__������������3λ��Ч���֣�ÿ�����ӵĵ���Ϊ1.6��10-19C������Ч��=ʵ�ʷ�Ӧ�������/��·��ͨ��������100%����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��XԪ������������Ӧ��ˮ����ΪH3XO4��������Ӧ����̬�⻯��Ϊ( )
A. HXB. H2XC. XH3D. XH4
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com