
°æƒø°ø∏ı∫œΩ”–÷ÿ“™µƒ”√Õ棨¥”∆‰∑œ¡œ÷–÷∆»°∏ıµƒ¡˜≥ûÁœ¬:
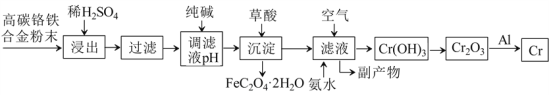
“—÷™: ¢Ÿ Cr+H2SO4=CrSO4+H2°¸£¨
¢⁄ ¡˜≥Ã÷–∏ı‘™ÀÿΩ˛≥ˆ÷Æ∫Û÷¡…˙≥…Cr(OH)3÷ƺ‰æ˘“‘◊‘”…“∆∂Ø¿Î◊”◊¥Ã¨¥Ê‘⁄”⁄»Ð“∫÷–°£
«Îªÿ¥œ¬¡–Œ Â:
£®1£©œ°¡ÚÀ·À·Ω˛π˝≥Ã÷–£¨Ã·∏þ°∞Ω˛≥ˆ¬ °±µƒ¥Î ©”–£∫________________________ (–¥“ªÃıº¥ø…) °£
£®2£©”√¥øºÓµ˜Ω⁄¬À“∫pH£¨µ√µΩƒ≥»ıºÓ≥¡µÌ£¨»Ù¥øºÓπ˝¡ø£¨‘Úø…ƒÐµº÷¬µƒ∫Ûπ˚ «_£∫_____________°£
£®3£©¡˜≥Ã÷–µƒ°∞∏±≤˙ŒÔ°±÷–£¨ø…”√◊˜ø…»Ð–‘±µ—Œ÷–∂æΩ‚∂溡µƒŒÔ÷ µƒªØ—ß Ω «___________£ªø…”√◊˜ªØ∑ µƒŒÔ÷ µƒªØ—ß Ω «_____________°£
£®4£©º”»Î≤ðÀ· µœ÷≥¡µÌ◊™ªØ∑¥”¶ªØ—ß∑Ω≥Ã ΩŒ™£∫_______________________________________°£
£®5£©¡˜≥Ã÷–¿˚”√¬¡»»∑¥”¶“±¡∂∏ıµƒªØ—ß∑Ω≥Ã ΩŒ™£∫_____________________________________°£
£®6£©¡˜≥Ã÷–”…¬À“∫…˙≥…Cr(OH)3µƒªØ—ß∑Ω≥Ã ΩŒ™£∫_____________________________________°£
£®7£©≥˝“—÷™∑¥”¶¢Ÿ÷ÆÕ‚£¨’˚∏ˆ¡˜≥Ã÷–…ʺ∞µƒ÷˜“™—ıªØªπ‘≠∑¥”¶”–_____∏ˆ£¨∑÷Ω‚∑¥”¶”–____∏ˆ°£
°æ¥∞∏°øº”»»°¢Ω¡∞Ë°¢ µ±Ã·∏þœ°¡ÚÀ·≈®∂»µ»(¥∞∏∫œ¿Ìº¥ø…) H2C2O4µƒœ˚∫ƒ¡øπ˝¥Û£®ªÚ πCr2+◊™ªØ≥…≥¡µÌ∂¯À∫ƒ£© Na2SO4 (NH4)2SO4 Fe(OH)2+H2C2O4=FeC2O4°§2H2O Cr2O3+2Al![]() Al2O3+2Cr 4CrSO4+O2+8NH3°§H2O+2H2O=4Cr(OH)3°˝+4(NH4)2SO4 3 1
Al2O3+2Cr 4CrSO4+O2+8NH3°§H2O+2H2O=4Cr(OH)3°˝+4(NH4)2SO4 3 1
°æΩ‚Œˆ°ø
∏þú∏ıÃ˙∫œΩ∑€ƒ©º”»Îœ°H2SO4Ω˛»°£¨∆‰÷–Cr°¢Fe»ÐΩ‚…˙≥…CrSO4°¢FeSO4£ªπ˝¬À∫Û”√¥øºÓµ˜Ω⁄pH πFe2+◊™ªØ≥…Fe£®OH£©2≥¡µÌ£¨‘Ÿº”»Î≤ðÀ·Ω´Fe£®OH£©2◊™ªØŒ™FeC2O4°§2H2O≥¡µÌ∂¯≥˝»•£ª¥À ±¬À“∫÷–÷˜“™∫¨CrSO4∫ÕNa2SO4£¨œÚ¬À“∫÷–Õ®»Îø’∆¯≤¢º”»Î∞±ÀÆ£¨¬À“∫÷–Cr2+◊™ªØ≥…Cr£®OH£©3≥¡µÌ£¨∑¢…˙µƒ∑¥”¶Œ™4CrSO4+O2+8NH3°§H2O+2H2O£Ω4Cr£®OH£©3°˝+4£®NH4£©2SO4£¨µ√µΩµƒ∏±≤˙ŒÔ÷˜“™∫¨£®NH4£©2SO4°¢Na2SO4£ªCr£®OH£©3 л»∑÷Ω‚≥…Cr2O3£ªCr2O3”ÎAl∑¢…˙¬¡»»∑¥”¶…˙≥…Cr∫ÕAl2O3£¨æð¥À∑÷Œˆ◊˜¥°£
£®1£©∏˘æðÕ‚ΩÁÃıº˛∂‘∑¥”¶ÀŸ¬ µƒ”∞œÏø…÷™œ°H2SO4À·Ω˛π˝≥Ã÷–£¨Ã·∏þ°∞Ω˛≥ˆ¬ °±µƒ¥Î ©”–£∫º”»»°¢Ω¡∞Ë°¢ µ±Ã·∏þœ°H2SO4µƒ≈®∂»µ»°£
£®2£©”√¥øºÓµ˜Ω⁄¬À“∫pH£¨ πFe2+◊™ªØ≥…Fe£®OH£©2≥¡µÌ£¨‘Ÿº”»Î≤ðÀ·Ω´Fe£®OH£©2◊™ªØŒ™FeC2O4°§2H2O≥¡µÌ∂¯≥˝»•°£»Ù¥øºÓπ˝¡ø£¨ø…ƒÐµº÷¬µƒ∫Ûπ˚ «£∫“ª∑Ω√Ê πCr2+◊™ªØ≥…≥¡µÌ∂¯±ªœ˚∫ƒ£¨ πCrµƒ≤˙¬ ΩµµÕ£ª¡Ì“ª∑Ω√Ê π∫Û–¯π˝≥Ã÷–H2C2O4µƒœ˚∫ƒ¡øπ˝¥Û°£
£®3£©≥˝»•Fe2+∫Ûµƒ¬À“∫÷–÷˜“™»Ð÷ Œ™CrSO4∫ÕNa2SO4£¨œÚ¬À“∫÷–Õ®»Îø’∆¯≤¢º”»Î∞±ÀÆ£¨¬À“∫÷–Cr2+◊™ªØ≥…Cr£®OH£©3≥¡µÌ£¨µ√µΩµƒ∏±≤˙ŒÔŒ™£®NH4£©2SO4∫ÕNa2SO4£¨∆‰÷–ø…”√◊˜ø…»Ð–‘±µ—Œ÷–∂æΩ‚∂溡µƒŒÔ÷ µƒªØ—ß Ω «Na2SO4£¨Ω‚∂浃‘≠¿ÌŒ™SO42-+Ba2+=BaSO4°˝°£ø…”√◊˜ªØ∑ µƒŒÔ÷ µƒªØ—ß Ω «£®NH4£©2SO4£¨£®NH4£©2SO4 «“ª÷÷µ™∑ °£
£®4£©º”»Î≤ðÀ·Ω´Fe£®OH£©2≥¡µÌ◊™ªØŒ™FeC2O4°§2H2O£¨≥¡µÌ◊™ªØ∑¥”¶µƒªØ—ß∑Ω≥Ã ΩŒ™Fe£®OH£©2+H2C2O4£ΩFeC2O4°§2H2O°£
£®5£©Al”ÎCr2O3∑¢…˙¬¡»»∑¥”¶…˙≥…Al2O3∫ÕCr£¨∑¥”¶µƒªØ—ß∑Ω≥Ã ΩŒ™2Al+Cr2O3![]() Al2O3+2Cr°££©¬À“∫÷–Õ®»Îø’∆¯°¢º”»Î∞±ÀÆCrSO4◊™ªØŒ™Cr£®OH£©3≥¡µÌ£¨1molCr2+ ß»•1molµÁ◊”…˙≥…1molCr£®OH£©3£¨1molO2µ√µΩ4molµÁ◊”£¨∏˘æðµ√ ßµÁ◊” ÿ∫„°¢‘≠◊” ÿ∫„£¨”…¬À“∫…˙≥…Cr£®OH£©3µƒªØ—ß∑Ω≥Ã ΩŒ™4CrSO4+O2+8NH3°§H2O+2H2O£Ω4Cr£®OH£©3°˝+4£®NH4£©2SO4°£
Al2O3+2Cr°££©¬À“∫÷–Õ®»Îø’∆¯°¢º”»Î∞±ÀÆCrSO4◊™ªØŒ™Cr£®OH£©3≥¡µÌ£¨1molCr2+ ß»•1molµÁ◊”…˙≥…1molCr£®OH£©3£¨1molO2µ√µΩ4molµÁ◊”£¨∏˘æðµ√ ßµÁ◊” ÿ∫„°¢‘≠◊” ÿ∫„£¨”…¬À“∫…˙≥…Cr£®OH£©3µƒªØ—ß∑Ω≥Ã ΩŒ™4CrSO4+O2+8NH3°§H2O+2H2O£Ω4Cr£®OH£©3°˝+4£®NH4£©2SO4°£
£®6£©≥˝∑¥”¶¢Ÿ÷ÆÕ‚£¨’˚∏ˆ¡˜≥Ã÷–…ʺ∞µƒ÷˜“™—ıªØªπ‘≠∑¥”¶ªπ”–£∫Fe+H2SO4£ΩFeSO4+H2°¸°¢4CrSO![]() Al2O3+2Cr£¨º¥—ıªØªπ‘≠∑¥”¶”–3∏ˆ£ª∑÷Ω‚∑¥”¶Œ™2Cr£®OH£©3
Al2O3+2Cr£¨º¥—ıªØªπ‘≠∑¥”¶”–3∏ˆ£ª∑÷Ω‚∑¥”¶Œ™2Cr£®OH£©3![]() Cr2O3+3H2O£¨º¥∑÷Ω‚∑¥”¶”–1∏ˆ°£
Cr2O3+3H2O£¨º¥∑÷Ω‚∑¥”¶”–1∏ˆ°£



| ƒÍº∂ | ∏þ÷–øŒ≥à | ƒÍº∂ | ≥ı÷–øŒ≥à |
| ∏þ“ª | ∏þ“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı“ª | ≥ı“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ∂˛ | ∏þ∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı∂˛ | ≥ı∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ»˝ | ∏þ»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı»˝ | ≥ı»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øœ¬±Ì ˝æð «∂‘”¶ŒÔ÷ µƒ»€µ„£∫
±ý∫≈ | ¢Ÿ | ¢⁄ | ¢€ | ¢Ð | ¢ð | ¢Þ | ¢þ | ¢ý |
ŒÔ÷ | Na2O | NaCl | AlF3 | AlCl3 | BCl3 | Al2O3 | CO2 | SiO2 |
»€µ„°Ê | 920 | 801 | 1291 | 160 | -107 | 2072 | -57 | 1723 |
£®1£©…œ ˆ…ʺ∞‘≠◊”÷–◊ÓªÓ∆√∑«Ω Ù‘≠◊”∫ÀÕ‚µÁ◊”≈≈≤º Ω «________________£ªƒ≥“ı¿Î◊”µƒπϵ¿±Ì æ ΩŒ™![]() £¨∆‰∫ÀÕ‚µÁ◊”’º”–µƒπϵ¿◊Ð ˝ «_____∏ˆ£¨”–______÷÷ƒÐ¡ø≤ªÕ¨µƒµÁ◊”£¨”–_____÷÷≤ªÕ¨‘À∂Ø◊¥Ã¨µƒµÁ◊”°£
£¨∆‰∫ÀÕ‚µÁ◊”’º”–µƒπϵ¿◊Ð ˝ «_____∏ˆ£¨”–______÷÷ƒÐ¡ø≤ªÕ¨µƒµÁ◊”£¨”–_____÷÷≤ªÕ¨‘À∂Ø◊¥Ã¨µƒµÁ◊”°£
£®2£©ŒÔ÷ ¢ŸµƒµÁ◊” Ω£∫____________£¨¢þµƒΩ·ππ Ω£∫_______________°£
£®3£©¢Ð»Ð”⁄ÀƻГ∫≥ À·–‘£¨”√¿Î◊”∑Ω≥Ã Ω±Ì æ∆‰‘≠“Ú_______________________________£ª»Ù∞—∆‰»Ð“∫º”»»’Ù∏…≤¢◊∆…’£¨µ√µΩµƒπÃà«_______________°£
£®4£©≤ªƒÐ”√”⁄±»ΩœNa”ÎAlΩ Ù–‘œý∂‘«ø»ıµƒ ¬ µ «_________________°£
A.◊Ó∏þº€—ıªØŒÔ∂‘”¶Àƪ،ԵƒºÓ–‘ B.Na◊ÓÕ‚≤„1∏ˆµÁ◊”∂¯Al ◊ÓÕ‚≤„3∏ˆµÁ◊”
C.µ•÷ ”ÎH2O∑¥”¶µƒƒ—“◊≥Ã∂» D.±»ΩœÕ¨≈®∂»NaCl∫ÕAlCl3µƒpH÷µ
£®5£©¢ý±»¢þ»€µ„∏þ≥ˆ∫Ð∂ý£¨∆‰¿Ì”… «£∫_____________________________£ª¢Ÿ∫Õ¢⁄∂º Ù”⁄¿Î◊”æß㨵´¢Ÿ±»¢⁄µƒ»€µ„∏þ£¨«ÎΩ‚ Õ‘≠“Ú____________________________°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø“ª∂®Ãıº˛œ¬£¨∂‘”⁄ø…ƒÊ∑¥”¶X(g)£´3Y(g)![]() 2Z(g)£¨»ÙX°¢Y°¢Zµƒ∆ º≈®∂»∑÷±Œ™c0(X)°¢c0(Y)°¢c0(Z)(æ˘≤ªŒ™¡„)£¨¥ÔµΩ∆Ω∫‚ ±£¨X°¢Y°¢Zµƒ≈®∂»∑÷±Œ™0.1 mol°§L£≠1°¢0.3 mol°§L£≠1°¢0.08 mol°§L£≠1£¨‘Úœ¬¡–≈–∂œ’˝»∑µƒ «(°°°°)
2Z(g)£¨»ÙX°¢Y°¢Zµƒ∆ º≈®∂»∑÷±Œ™c0(X)°¢c0(Y)°¢c0(Z)(æ˘≤ªŒ™¡„)£¨¥ÔµΩ∆Ω∫‚ ±£¨X°¢Y°¢Zµƒ≈®∂»∑÷±Œ™0.1 mol°§L£≠1°¢0.3 mol°§L£≠1°¢0.08 mol°§L£≠1£¨‘Úœ¬¡–≈–∂œ’˝»∑µƒ «(°°°°)
A. c0(X)°√c0(Y)£Ω3°√1
B. ∆Ω∫‚ ±£¨Y∫ÕZµƒ…˙≥…ÀŸ¬ ÷Ʊ»Œ™2°√3
C. X°¢Yµƒ◊™ªØ¬ ≤ªœýµ»
D. c0(X)µƒ»°÷µ∑∂ŒßŒ™0 mol°§L£≠1<c0(X)<0.14 mol°§L£≠1
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø”√“ı¿Î◊”Ωªªªƒ§øÿ÷∆µÁΩ‚“∫÷–OH£≠µƒ≈®∂»÷∆±∏ƒ…√◊Cu2O£¨∑¥”¶Œ™2Cu£´H2O![]() Cu2O£´H2°¸£¨◊∞÷√»ÁÕº£¨œ¬¡–Àµ∑®÷–’˝»∑µƒ «
Cu2O£´H2°¸£¨◊∞÷√»ÁÕº£¨œ¬¡–Àµ∑®÷–’˝»∑µƒ «
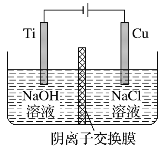
A. µÁΩ‚ ±Cl-Õ®π˝Ωªªªƒ§œÚTiº´“∆∂Ø
B. —Ùº´∑¢…˙µƒ∑¥”¶Œ™£∫2Cu £≠2e£≠ +2OH£≠ = Cu2O+H2O
C. “ıº´OH-∑≈µÁ£¨”–O2…˙≥…
D. TiµÁº´∫ÕCuµÁº´…˙≥…ŒÔŒÔ÷ µƒ¡ø÷Ʊ»Œ™2°√1
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øŒ™¡Àºı…Ÿ≥« –ø’∆¯Œ€»æ£¨“™«Û π”√ŒÞ«¶∆˚”Õ£¨À˘ŒΩŒÞ«¶∆˚”Õ «÷∏£® £©
A.≤ª”√«¶Õ∞◊∞µƒ∆˚”Õ
B.≤ª∫¨Àƒ““ª˘«¶µƒ∆˚”Õ
C.≤ª∫¨Pb(NO3£©2µƒ∆˚”Õ
D.≤ª∫¨—ıªØ«¶µƒ∆˚”Õ
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø∂‘Ù«ª˘±Ω““À· «∫œ≥…“©ŒÔµƒ÷–º‰Ã£¨∆‰÷∆±∏¬∑œþ»Áœ¬£®AŒ™∑ºœ„Ã˛£©£∫
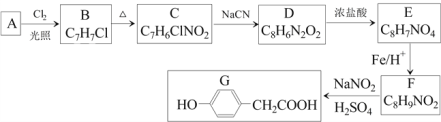
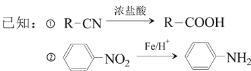
ªÿ¥œ¬¡–Œ Â:
£®1£©Aµƒ√˚≥∆ «______________°£
£®2£©B°˙Cµƒ∑¥”¶ ‘º¡ «_______ £¨∑¥”¶¿ý–Õ «_______£ªE°˙Fµƒ∑¥”¶¿ý–Õ «_______°£
£®3£©C°˙D∑¥”¶µƒªØ—ß∑Ω≥Ã ΩŒ™________________°£
£®4£©E÷–∫¨—ıπŸƒÐÕ≈µƒ√˚≥∆Œ™______°£
£®5£©1molG”Î◊„¡øNaOH»Ð“∫∑¥”¶£¨ø…“‘œ˚∫ƒ_____molNaOH°£
£®6£©H «GµƒÕ¨œµŒÔ£¨¬˙◊„œ¬¡–Ãıº˛µƒHµƒÕ¨∑÷“ÏππÔ–_______÷÷£®≤ªøº¬«¡¢Ã“Ïπ𣩰£
¢Ÿ Hœý∂‘∑÷◊”÷ ¡ø±»G¥Û14 ¢⁄ ±Ωª∑…œ∫¨¡Ω∏ˆ»°¥˙ª˘
∆‰÷–∫À¥≈π≤’Ò«‚∆◊Œ™¡˘◊È∑£¨∑Â√ʪ˝÷Ʊ»Œ™1:2:2:2:2:1µƒΩ·ππºÚ ΩŒ™_____________°£
£®7£©Ω·∫œ“‘…œ∫œ≥…¬∑œþº∞œýπÿ–≈œ¢£¨…˺∆”…±Ω÷∆±∏±Ω∑”µƒ∫œ≥…¬∑œþ________________°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø√Ð∂»Œ™0.91g/cm3µƒ∞±ÀÆ£¨÷ ¡ø∑÷ ˝Œ™25%£¨∏√∞±ÀÆ”√µ»Ãª˝µƒÀÆœ° Õ∫Û£¨À˘µ√»Ð“∫»Ð÷ ÷ ¡ø∑÷ ˝Œ™
A. 12.5%B. >12.5%C. <12.5%D. ŒÞ∑®»∑∂®
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øÓ—“±¡∂≥ߔά»ºÓ≥ß°¢º◊¥º≥ß◊È≥…“ª∏ˆ≤˙“µ¡¥(»ÁÕºÀ˘ æ)£¨Ω´¥Û¥Û÷∏þ◊ ‘¥µƒ¿˚”√¬ £¨ºı…Ÿª∑æ≥Œ€»æ°£

«Îªÿ¥œ¬¡–Œ Â:
(1)FeŒª”⁄‘™Àÿ÷Ð∆⁄±Ì÷–µƒŒª÷√ «__________°£
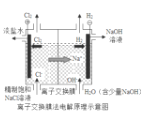
(2)–¥≥ˆ¡˜≥Ã÷–µÁΩ‚≥ÿ¿Ô∑¢…˙∑¥”¶µƒ¿Î◊”∑Ω≥à Ω: __________°£
(3)–¥≥ˆ¡˜≥Ã÷–°∞¬»ªØ°±µƒªØ—ß∑Ω≥à Ω: __________°£
(4)–¥≥ˆTiCl4ÕÍ»´ÀÆΩ‚…˙≥…TiO2°§H2OµƒªØ—ß∑Ω≥à Ω: __________°£
(5)∏þŒ¬¬Ø÷–Õ®»ÎArµƒ◊˜”√ «___________°£
(6)…œ ˆ¡˜≥Ã÷–ø…—≠ª∑¿˚”√µƒŒÔ÷ ”–__________°£
(7)œ¬ÕºŒ™¬»ºÓ𧓵µƒ◊∞÷√ æ“‚Õº£¨ π”√______(ÃÓ°∞“ı°±ªÚ°∞—Ù°±)¿Î◊”Ωªªªƒ§£¨ƒøµƒ≥˝¡ÀΩµµÕ∑÷¿Î«‚—ıªØƒ∆µƒ≥…±æÕ‚ªπø…“‘________°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øº”»Î…Ÿ–Ìœ¬¡–“ª÷÷ŒÔ÷ £¨≤ªƒÐ π‰ÂÀÆ—’…´±‰«≥µƒ «
A.Mg∑€B.H2S(g)C.KI(s)D.CCl4
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
π˙º —ß–£”≈—° - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒÞ÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com