
�л���A���������Ƿ��͵õ���Ҳ�ɴ���ţ������ȡ��������AΪ��ɫ��Һ�壬������ˮ��Ϊ�о�A�������ṹ������������ʵ�飺
| ʵ�鲽�� | ���ͻ�ʵ����� |
| �ų�ȡA 9.0 g������ʹ�������������ܶ�����ͬ������H2��45���� | ��ͨ��������գ� ��A����Է�������Ϊ�� �� |
| �ƽ���9.0 g A��������O2�г��ȼ�գ���ʹ��������λ���ͨ��Ũ���ᡢ��ʯ�ң��������߷ֱ�����5.4 g��13.2 g | ��A�ķ���ʽΪ�� �� |
| ����ȡA 9.0 g����������NaHCO3��ĩ��Ӧ������2.24 L CO2(��״��)���������������Ʒ�Ӧ������2.24 L H2(��״��) | ��A�й����ŵĽṹ��ʽ�� �� �� |
��A�ĺ˴Ź�����������ͼ�� | ��A�к��� ����ԭ�� |
| ������������A�Ľṹ��ʽ ��A��ŨH2SO4��ϣ���һ�������·�Ӧ������Ԫ��״��B��B�Ľṹ��ʽ �� | |
( ÿ��1�ֹ�7��)��90�� ��C3H6O3�� ���Ȼ����ǻ��� ��4��
��A�Ľṹ��ʽ�� B�Ľṹ��ʽ��
B�Ľṹ��ʽ��
���������������1�����л��������ܶ�����ͬ������H2��45�������л������Է�������Ϊ45��2��90��
��2��9.0gA�����ʵ�����0.1mol��Ũ����ͼ�ʯ�����ӵ������ֱ���ˮ��CO2����������������ˮ��5.4g��CO2��13.2g�����ʵ����ֱ���5.4g��18g/mol��0.3mol��13.2g��44g/mol��0.3mol�������л���A��̼��ԭ�ӵĸ����ֱ���3����6��������ԭ�ӵĸ����� ������A�Ļ�ѧʽ��C3H6O3��
������A�Ļ�ѧʽ��C3H6O3��
��3��A��������NaHCO3��ĩ��Ӧ������2.24LCO2����״������˵���л���A�к����Ȼ���9.0g�л���A�����ʵ���Ϊ0.1mol��������̼�����ʵ���Ϊ2.24L��22.4L/mol��0.1mol���Ȼ���̼�����ư�1��1��Ӧ���ʷ����к����Ȼ���ĿΪ0.1mol��0.1mol��1����2.24LH2����״���������ʵ���Ϊ2.24L��22.4L/mol��0.1mol��0.1mol�Ȼ����Ʒ�Ӧ����0.05molH2�����л���A�л������ǻ������к����ǻ���ĿΪ ��
��
��4���ؼ��˴Ź�������֪��A�����к���4����ԭ�ӡ�
��5������������A�Ľṹ��ʽ��CH3CH��OH��COOH���л���A�к����Ȼ����ǻ���������������������Ӧ������Ԫ����B�����B�Ľṹ��ʽ�� ��
��
���㣺�����л������ʽ��ȷ��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������߿����ۺ���ǿ����ע�ض�ѧ������֪ʶ������ѵ����ͬʱ�����ض�ѧ����������������ⷽ����ָ����ѵ�������������ܽ�ȫ��ؿ���ѧ�����л���ѧ����֪ʶ����˼ά����������˼ά���������ѧ����Ӧ�������ʹ���Ч�ʣ�Ҳ����������ѧ������ѧ������֪ʶ��Ǩ������������Ĺؼ��Ǽ�ס���������ŵĽṹ�������Լ�������֮����ת����Ȼ��������������ü��ɡ�����ʱ��Ҫע�������غ㷽��ȷ��ԭ����Ŀ��


 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
14��)A��J�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮�����ϵ����ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬����� A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
����д���пհף�
��1��A��B��C��D��E��F��������������ͬһ��Ԫ�������ڱ���λ���� ��
��2������C��Һ�������ӷ����ǣ�д�������������ۣ�
��
��3��д����Ӧ�ٻ�ѧ����ʽ�� ��
��4��д����Ӧ�����ӷ���ʽ�� ��
��5��д����Ӧ��ѧ����ʽ�� ��
��6��д����Ӧ�������ĵ缫��Ӧʽ�� ��
��7���������仯�Ƕȿ�����Ӧ�٢ڢ��У����ڡ�H��0�ķ�Ӧ�� ������ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��15�֣�������F�Ǻϳ�������Ѫ�ܲ�����Ҫҩ��������İ����м��塣����һ�ֺϳ�·�����£�
�ش��������⣺
��1��A�Ļ�ѧ����Ϊ �� ��2�� B����C�ķ�Ӧ����Ϊ ��
��3����C����D�Ļ�ѧ����ʽΪ�� ����4��E�ķ���ʽΪ ��
��5��F�Ľṹ��ʽΪ ��1mol F�����������Ʒ�Ӧ�����������ڱ�״���µ����Ϊ ��
��6��B�ж���ͬ���칹�壬д��ͬʱ��������������B������ͬ���칹��Ľṹ��ʽ ��
�ټ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ��
�ں˴Ź�������ͼ��4�����շ塢�ҷ����֮��Ϊ1��2��6��3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
(17��)����ȩ( )��һ���������ϣ��㷺Ӧ�������ϡ�ҽҩ��Ⱦ�ϼ�ũҩ����ҵ�������ǹ�ҵ���Ա�Ϊ��Ҫԭ�ϣ���������ȩ��һ�ֺϳ�·��(���ַ�Ӧ����������������ȥ)��
)��һ���������ϣ��㷺Ӧ�������ϡ�ҽҩ��Ⱦ�ϼ�ũҩ����ҵ�������ǹ�ҵ���Ա�Ϊ��Ҫԭ�ϣ���������ȩ��һ�ֺϳ�·��(���ַ�Ӧ����������������ȥ)��
��ش��������⣺
��1����֪��A�Ľṹ��ʽΪ ���˴Ź����������� ���塣B�����ֿ��ܵĽṹ��д�����к�������̼��B�Ľṹ��ʽ�� (����̼�á�*����ע)��
���˴Ź����������� ���塣B�����ֿ��ܵĽṹ��д�����к�������̼��B�Ľṹ��ʽ�� (����̼�á�*����ע)��
��2�������ϳ�·���У���Ӧ�۵ķ�Ӧ����Ϊ ����Ӧ�ݵ�����Ϊ ��
��3����Ӧ�Ļ�ѧ����ʽΪ ��
��4������ȩ���ж���ͬ���칹�壬����ijЩ����������������
a����ˮ��Һ��FeCl3��Һ����ɫ��
b��������ֻ������ȡ�������ұ����ϵ�һ����������֣�
c��������û�м���
д�������������������ʿ��ܵĽṹ��ʽ�� ��ֻҪ��дһ�֣���
��5����������������ٴ��ϳ��õĿ�����ʧ��ҩ����ṹ��ʽ�� ��д����1-����Ϊԭ���Ʊ�������������ĺϳ�·������ͼ�����Լ����ã���
��д����1-����Ϊԭ���Ʊ�������������ĺϳ�·������ͼ�����Լ����ã���
��֪��CH2��CHCH3+Cl2 CH2��CHCH2Cl+HCl
CH2��CHCH2Cl+HCl
�ϳ�·������ͼʾ�����£�CH3CH2OH CH2��CH2
CH2��CH2

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
(16��)�ڶ�����Ӧ��ż����Ӧ��һ�֣�����ʵ��±�����뺬���ӻ��ķ�Ӧ�����磺
��Ӧ��
��1���������ķ���ʽΪ_________________________��
��2����������ǻ�������ͬϵ�����ʽΪC8H9Br����Ϊ���Ķ�λ��ȡ�����˴Ź�������ͼ����4��壬�������Ϊ2��2��2��3����ṹ��ʽΪ_____________(��дһ��)��
��3��1mol������������____molH2�����ӳɷ�Ӧ������ṹ��ʽΪ______________��
��4�����������һ��ͬ���칹����Ľṹ��ʽΪ �������������ƴ���Һ���������·�Ӧ�ķ���ʽΪ:___________________________________________��
�������������ƴ���Һ���������·�Ӧ�ķ���ʽΪ:___________________________________________��
��5��һ�������£� ��
�� Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���μӷ�Ӧ�ķ�����Ϊ2��1�������ɵIJ���Ľṹ��ʽΪ_________________��
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���μӷ�Ӧ�ķ�����Ϊ2��1�������ɵIJ���Ľṹ��ʽΪ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�����ӻ�����ҩ���ƹ����з��ֵ�һ����Ҫ�������ʣ����п����ʡ���������������ȱѪ�����ʡ�������ij�о�С�������һ�������ӻ������H�ĺϳ�·�ߣ�
(1)ԭ��A��ͬ���칹���У����б������Һ˴Ź�����������4�������______(д����ṹ��ʽ����
(2)�۵ķ�Ӧ������______��ԭ��D�к��еĹ�����������______��______��
(3)��Ӧ�ڵĻ�ѧ����ʽ��______________________________��
(4)ԭ��B��������������,����ÿ����(˳��ϩ��� ��������,�����Ծ����б仯�ֱ�õ�ƻ���ᣨ
��������,�����Ծ����б仯�ֱ�õ�ƻ���ᣨ ���;ۺ���Q:
���;ۺ���Q:
�ٰ뷽����ԭ��B��ͬ���칹�壬�����к�1����(��Ԫ̼������1���ǻ��������� ��O��O�������뷽��Ľṹ��ʽ��____________��
��д����ӦI�ͷ�ӦII�Ļ�ѧ����ʽ_____________��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�л��������������洦�ɼ�������ǿ����ҩ������ʵ�ij�ֺϳ�·�����£� 
��ش��������⣺
��1�������ķ���ʽ�ǣ� ��
��2������˵����ȷ���� ������ţ���
a����Ӧ����������Ӧ��
b������������ϩȩ��A��B����������Ʒ�Ӧ�ų�������
c������A��B��E��������ˮ�����ӳɷ�Ӧ����ɫ��
d���л��������Է���ˮ�ⷴӦ��
��3������B���� ������ţ���
a������ b��ϩ�� c���� d���� e�� ��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
��5����Ӧ�IJ���Ϊ������FeCl2�� ���ڴ��������£�����������ӳɺ�IJ���Ϊ��д�ṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
(12��)ij�������A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫�����˴Ź����ױ���������ֻ��һ�����͵��⣮
��1��A�Ľṹ��ʽΪ ��
��2��A�е�̼ԭ���Ƿ���ͬһƽ�棿 (��ǡ����ǡ�)��
��3������ͼ�У�D1��D2��Ϊͬ���칹�壬E1��E2��Ϊͬ���칹�壮
��Ӧ�ڵĻ�ѧ����ʽΪ ��
C�Ļ�ѧ����Ϊ ��E2�Ľṹ��ʽ�� ��
�ܡ��ķ�Ӧ���������� , ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
���ɱ��������ȩ�����ʺϳ�F���ϳ�·�����£�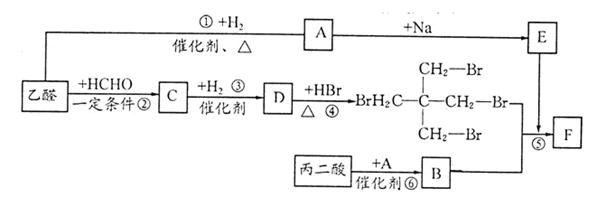

��ش��������⣺
��1��A�Ĺ����ŵ�����Ϊ ������ϳɷ��������Ʋ�C�Ľṹ��ʽ ��
��2��д����Ӧ�ܵĻ�ѧ����ʽ ����Ӧ����Ϊ ��
��3��д����������������D��ͬ���칹��Ľṹ��ʽ ��
����D������ȫ��ͬ�Ĺ�����
��ÿ��̼�����ֻ����һ��������
�ۺ˴Ź���������5�����շ�
��4����E���ڷ�̪ϡ��Һ�����Թ۲쵽�������� ����֪F���ʺɱ����ֵΪ384����E�������ºϳ�F�Ļ�ѧ��Ӧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com