
��15�֣�������F�Ǻϳ�������Ѫ�ܲ�����Ҫҩ��������İ����м��塣����һ�ֺϳ�·�����£�
�ش��������⣺
��1��A�Ļ�ѧ����Ϊ �� ��2�� B����C�ķ�Ӧ����Ϊ ��
��3����C����D�Ļ�ѧ����ʽΪ�� ����4��E�ķ���ʽΪ ��
��5��F�Ľṹ��ʽΪ ��1mol F�����������Ʒ�Ӧ�����������ڱ�״���µ����Ϊ ��
��6��B�ж���ͬ���칹�壬д��ͬʱ��������������B������ͬ���칹��Ľṹ��ʽ ��
�ټ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ��
�ں˴Ź�������ͼ��4�����շ塢�ҷ����֮��Ϊ1��2��6��3��
��1�����Ҵ�����2��ȡ����Ӧ����3��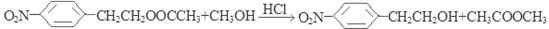 ����4��C8H11ON����5��
����4��C8H11ON����5�� ��22.4L����6��
��22.4L����6�� ��
�� ��
��
���������������1��A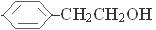 �Ļ�ѧ����Ϊ���Ҵ�����2��A��CH3COCl����ȡ����Ӧ�õ�B��
�Ļ�ѧ����Ϊ���Ҵ�����2��A��CH3COCl����ȡ����Ӧ�õ�B��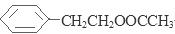 ��HCl��B��Ũ���ᷢ��ȡ����Ӧ�õ�C��
��HCl��B��Ũ���ᷢ��ȡ����Ӧ�õ�C��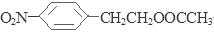 ����3��C��CH3OH��HCl�����·�����������Ӧ��Ҳ��ȡ����Ӧ���õ�D��
����3��C��CH3OH��HCl�����·�����������Ӧ��Ҳ��ȡ����Ӧ���õ�D��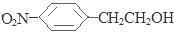 ��CH3COOCH3���÷�Ӧ�ķ���ʽΪ��
��CH3COOCH3���÷�Ӧ�ķ���ʽΪ��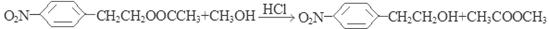 ����4��D��SnCl2��HCl�����±���ԭ�õ�E��
����4��D��SnCl2��HCl�����±���ԭ�õ�E��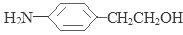 ��E��H2SO4��NaNO2������Ӧ�õ�F�����ǻ����Ҵ�
��E��H2SO4��NaNO2������Ӧ�õ�F�����ǻ����Ҵ�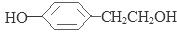 ������E�к��������ǻ������������Na������Ӧ������1mol F�����������Ʒ�Ӧ���������������ʵ���Ϊ1mol.�����ڱ�״���µ����22.4L����6���������������ټ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ���ں˴Ź�������ͼ��4�����շ塢�ҷ����֮��Ϊ1��2��6��3��B����ͬ���칹��Ľṹ��ʽ��
������E�к��������ǻ������������Na������Ӧ������1mol F�����������Ʒ�Ӧ���������������ʵ���Ϊ1mol.�����ڱ�״���µ����22.4L����6���������������ټ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ���ں˴Ź�������ͼ��4�����շ塢�ҷ����֮��Ϊ1��2��6��3��B����ͬ���칹��Ľṹ��ʽ��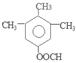 ��
�� ��
��
���㣺�������ʵĽṹ�����ʡ��ת������Ӧ���͡�ͬ���칹�弰��ѧ����ʽ����д��֪ʶ��


 �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
A��B��C��D��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮��Ĺ�ϵ����ͼ��ʾ�����ֲ�������ȥ����
��1����AΪ�������ʣ�D��ijǿ���ϡ��Һ����ӦB��A��C�����ӷ���ʽΪ ��
��2����A��BΪ�Σ�DΪǿ�A��ˮ��Һ�����ԣ���Ӧ A��D��B�����ӷ���ʽΪ ��
��3����AΪǿ�DΪ��̬�������������ʱ����B��ˮ��Һ¶���ڿ����У���pH��ʱ��t�仯������ͼa��ͼb��ʾ��������D���ܽ��ˮ�Ļӷ�����
����ͼa������ʵ����DΪ ���ѧʽ������ʱͼa��x 7�������������������
����ͼb������ʵ����ͼb��y��7��B����ɫ��ӦΪ��ɫ����B��Һ�и�����Ũ���ɴ�С��˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�����13�֣�
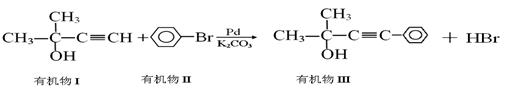
��������һ����������ʧ����ҩ����ĺϳ�·�����£�
47����B��C�ķ�Ӧ����Ϊ ��
48��д��A��Ũ��ˮ��Ӧ�Ļ�ѧ����ʽ ��
49����A�Ʊ�B�Ĺ�����������������E������B��Ϊͬ���칹�壬E�Ľṹ��ʽΪ
��
50��д��ͬʱ��������������D��һ��ͬ���칹��Ľṹ��ʽ ��
������ -�����
-�����
���DZ���������ұ����ϵ�һ�ȴ���ֻ�����֣�
�۷����к����������� 51����֪��ϩ�ڴ�����������������Ӧ�������ɻ������飨 ����д����
51����֪��ϩ�ڴ�����������������Ӧ�������ɻ������飨 ����д����
�ڼ�����( )���Ҵ�Ϊԭ���Ʊ�
)���Ҵ�Ϊԭ���Ʊ�  �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����ѧ��ѡ��5���л���ѧ��������15�֣�
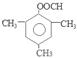
����ͬϵ��������������ʶ�ķ����廯������жԶ��ױ���Ӣ������p��xylene����дΪPX���ǻ�ѧ��ҵ����Ҫԭ�ϡ�
��1��д��PX�Ľṹ��ʽ______________��
��2��PX���ܷ����ķ�Ӧ��_____ __��___ _____�����Ӧ���ͣ�ֻ�����֡���
��3���л���M��������ͼ��ʾ��ת����ϵ������A ��PX��һ��ͬ���칹�塣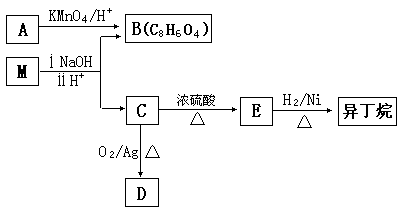
��֪��M�ķ���ʽΪC16H22O4��D�ܷ���������Ӧ��
��B�ı����ϴ���3 �ֲ�ͬ��ѧ��������ԭ�ӣ���B �Ľṹ��ʽ��__________��
��D�����������Ĺ�������______________���ѧʽ����
��M�Ľṹ��ʽ��_______________��
��4��F��B ��һ��ͬ���칹�壬��������������
a���DZ��Ķ�λ��ȡ���b����FeCl3��Һ��ʾ��ɫ��c������̼��������Һ��Ӧ��
д��F��NaHCO3��Һ��Ӧ�Ļ�ѧ����ʽ_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��14�֣�������������������E���������Ѫ֢֬�����Ʒ�����Ӧ��ǰ������ϳ�·�ߣ����ַ�Ӧ������ȥ�����£�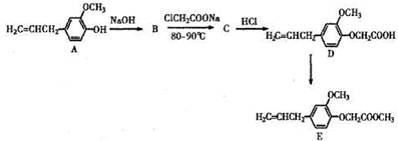
��1��B ��C�ķ�Ӧ������ ��B�Ľṹ��ʽ�� ����2��D�к��������ŵ��������ѻ��� ��
��3����д��ClCH2COONa���ữ������ͬʱ��������������ͬ���칹��ṹ��ʽ
���ܷ���������Ӧ ����������
��4����D�ϳ�E�Ļ�ѧ����ʽ�� ��
��5�����й���A��˵����ȷ���� ��
| A��1molA��ȫȼ������12molO2 | B����ʹ����KMnO4��Һ��ɫ |
| C������NaHCO3��Ӧ | D���������巢���ӳɷ�Ӧ���ܷ���ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��16�֣���֪B�Ǻϳɾ۶Ա��������Ҷ�������PET������Ҫԭ�ϣ�������ת����ϵ��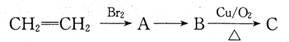
��1��B�Ĺ���������Ϊ ��
��2��A��B�ķ�Ӧ����ʽ�� ��
��3����ʢ��1ml������Һ�Ľྻ�Թ��е�������C����ˮԡ���ȿɹ۲쵽�������� ��
��4����ҵ�ϳ�PET�������£�
�� ��ϵͳ����Ϊ �������ķ�Ӧ����Ϊ ��
��ϵͳ����Ϊ �������ķ�Ӧ����Ϊ ��
���ò����õ�����Ϣ�������з���ʽ��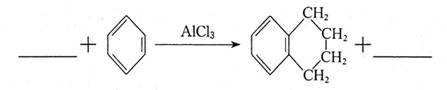
��ʵ�������ת���������Լ�Ϊ ��
��д����������������X������ͬ���칹�� ��
���������б�������������״�ṹ ������������Һ��ˮ�� ��1mol����������4molNaOH��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
������16�֣�
|
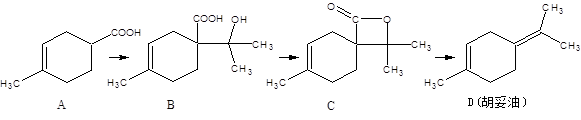
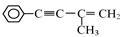 �ķ�Ӧ������ ��
�ķ�Ӧ������ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�л���A���������Ƿ��͵õ���Ҳ�ɴ���ţ������ȡ��������AΪ��ɫ��Һ�壬������ˮ��Ϊ�о�A�������ṹ������������ʵ�飺
| ʵ�鲽�� | ���ͻ�ʵ����� |
| �ų�ȡA 9.0 g������ʹ�������������ܶ�����ͬ������H2��45���� | ��ͨ��������գ� ��A����Է�������Ϊ�� �� |
| �ƽ���9.0 g A��������O2�г��ȼ�գ���ʹ��������λ���ͨ��Ũ���ᡢ��ʯ�ң��������߷ֱ�����5.4 g��13.2 g | ��A�ķ���ʽΪ�� �� |
| ����ȡA 9.0 g����������NaHCO3��ĩ��Ӧ������2.24 L CO2(��״��)���������������Ʒ�Ӧ������2.24 L H2(��״��) | ��A�й����ŵĽṹ��ʽ�� �� �� |
��A�ĺ˴Ź�����������ͼ�� | ��A�к��� ����ԭ�� |
| ������������A�Ľṹ��ʽ ��A��ŨH2SO4��ϣ���һ�������·�Ӧ������Ԫ��״��B��B�Ľṹ��ʽ �� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��12�֣�������������C3H6�ϳ��л��߷���E��C6H14������ͼ����ش��������⣺
��1��C6H14�˴Ź�����ֻ�����ַ壬��C6H14�Ľṹ��ʽΪ��_________________,
д��E�Ľṹ��ʽ��___________________��
��2��д��B������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ��________________________��
��3��D��ͬ���칹��ܶ࣬��������������ͬ���칹����__________��
�ٺ�̼̼˫�� ����ˮ�� ���ܷ���������Ӧ
��4����������ѧ֪ʶ����ͼ�������Ϣ�����Ҵ�Ϊ��Ҫԭ��ͨ���������ܺϳɻ�����(���Լ���ѡ����д����һ���͵�������ѧ��Ӧ�Ļ�ѧ����ʽ:
________________________________��________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com