
��12�֣�������������C3H6�ϳ��л��߷���E��C6H14������ͼ����ش��������⣺
��1��C6H14�˴Ź�����ֻ�����ַ壬��C6H14�Ľṹ��ʽΪ��_________________,
д��E�Ľṹ��ʽ��___________________��
��2��д��B������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ��________________________��
��3��D��ͬ���칹��ܶ࣬��������������ͬ���칹����__________��
�ٺ�̼̼˫�� ����ˮ�� ���ܷ���������Ӧ
��4����������ѧ֪ʶ����ͼ�������Ϣ�����Ҵ�Ϊ��Ҫԭ��ͨ���������ܺϳɻ�����(���Լ���ѡ����д����һ���͵�������ѧ��Ӧ�Ļ�ѧ����ʽ:
________________________________��________________________________________��
��1��(CH3)2CHCH(CH3)2��2�֣���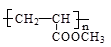 ��2�֣�
��2�֣�
��2��CH2��CHCHO + 2Cu(OH)2+NaOH��CH2��CHCOONa + Cu2O��+3H2O ��2�֣�
��3��3��2�֣�
��4��CH3CH2OH 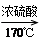 CH2��CH2�� + H2O��2�֣�
CH2��CH2�� + H2O��2�֣�
3BrCH2CH2Br +6 Na
 + 6NaBr��2�֣�
+ 6NaBr��2�֣�
��������������������������ͼ����Ӧ��Ϊȡ����Ӧ��CH2=CHCH3����CH2=CHCH2Cl����Ӧ��Ϊ±��������ȡ����Ӧ������A��CH2=CHCH2OH����Ӧ��Ϊ���Ĵ�����������B��CH2=CHCHO����Ӧ��Ϊȩ���������ữ������C��CH2=CHCOOH����Ӧ��Ϊ����ͼ״�����������D��CH2=CHCOOCH3��D��̼̼˫�������Ӿ۷�Ӧ����D�� ��CH2=CHCH3�����ķ�Ӧ��Ϊ�ӳɷ�Ӧ������ΪCH3CH2CH2Cl��CH3CHClCH3����Ӧ�߷���ȡ�����ò���C6H14�ĺ˴Ź�������ֻ��2�ַ壬��������ֻ������H����Ӧ�ļӳɲ���ΪCH3CHClCH3���õ���C6H14�ĽṹΪ��CH3CH��CH3��CH��CH3��CH3��
��CH2=CHCH3�����ķ�Ӧ��Ϊ�ӳɷ�Ӧ������ΪCH3CH2CH2Cl��CH3CHClCH3����Ӧ�߷���ȡ�����ò���C6H14�ĺ˴Ź�������ֻ��2�ַ壬��������ֻ������H����Ӧ�ļӳɲ���ΪCH3CHClCH3���õ���C6H14�ĽṹΪ��CH3CH��CH3��CH��CH3��CH3��
��3������Ҫ���D��ͬ���칹����3�֣�HCOOCH2CH=CH2��HCOOCH=CHCH3��CH2=C��CH3��OOCH���˴Ź������������ټ���ԭ���������ٵ���CH2=C��CH3��OOCH��
��4����������ͼ�з�Ӧ�ߵ����ͣ��Ҵ��ȷ�����ȥ��Ӧ������ϩ����ϩ��±�ص��ʼӳɵõ�±������±������±������Na�����¿����ɻ����顣
���㣺�����л���Ľṹ�����ʣ��л���Ӧ�����жϣ��л���ṹʽ��д���л���ѧ����ʽ��д��ͬ���칹����Ŀ���жϺ���д���л���ϳ���Ƶȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��15�֣�������F�Ǻϳ�������Ѫ�ܲ�����Ҫҩ��������İ����м��塣����һ�ֺϳ�·�����£�
�ش��������⣺
��1��A�Ļ�ѧ����Ϊ �� ��2�� B����C�ķ�Ӧ����Ϊ ��
��3����C����D�Ļ�ѧ����ʽΪ�� ����4��E�ķ���ʽΪ ��
��5��F�Ľṹ��ʽΪ ��1mol F�����������Ʒ�Ӧ�����������ڱ�״���µ����Ϊ ��
��6��B�ж���ͬ���칹�壬д��ͬʱ��������������B������ͬ���칹��Ľṹ��ʽ ��
�ټ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ��
�ں˴Ź�������ͼ��4�����շ塢�ҷ����֮��Ϊ1��2��6��3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�л��������������洦�ɼ�������ǿ����ҩ������ʵ�ij�ֺϳ�·�����£� 
��ش��������⣺
��1�������ķ���ʽ�ǣ� ��
��2������˵����ȷ���� ������ţ���
a����Ӧ����������Ӧ��
b������������ϩȩ��A��B����������Ʒ�Ӧ�ų�������
c������A��B��E��������ˮ�����ӳɷ�Ӧ����ɫ��
d���л��������Է���ˮ�ⷴӦ��
��3������B���� ������ţ���
a������ b��ϩ�� c���� d���� e�� ��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
��5����Ӧ�IJ���Ϊ������FeCl2�� ���ڴ��������£�����������ӳɺ�IJ���Ϊ��д�ṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
(12��)ij�������A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫�����˴Ź����ױ���������ֻ��һ�����͵��⣮
��1��A�Ľṹ��ʽΪ ��
��2��A�е�̼ԭ���Ƿ���ͬһƽ�棿 (��ǡ����ǡ�)��
��3������ͼ�У�D1��D2��Ϊͬ���칹�壬E1��E2��Ϊͬ���칹�壮
��Ӧ�ڵĻ�ѧ����ʽΪ ��
C�Ļ�ѧ����Ϊ ��E2�Ľṹ��ʽ�� ��
�ܡ��ķ�Ӧ���������� , ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����֪�������ǻ�ͬʱ����ͬһ̼ԭ���ϵĽṹ�Dz��ȶ��ģ�����������ˮ��Ӧ��
���з���ʽΪC9H8O2Br2������M����һ�������¿ɷ�������һϵ�з�Ӧ��
��֪���л���A����Է�������Ϊ44���л���Iֻ��һ�ֽṹ����ʹ���CCl4��Һ��ɫ����ش��������⣺
��1��G��H�ķ�Ӧ������ ��
��2��H�еĹ����ŵ�����Ϊ ��D�Ľṹ��ʽΪ ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��A�� B�� ��
��H�� I�� ��
��4����G��Ϊͬ���칹�壬������������ȡ����������FeCl3��Һ��ɫ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
������A�Ǻϳ���Ȼ�ĵ��壬����ʽΪC5H8��A��һϵ�з�Ӧ����(���ַ�Ӧ������ȥ)��
�ش��������⣺
(1)A�Ľṹ��ʽΪ ����ѧ������ ��
(2)B�ķ���ʽΪ ��
(3)�ڵķ�Ӧ����ʽΪ ��
(4)�ٺ͢۵ķ�Ӧ���ͷֱ��� �� ��
(5)CΪ�������������������Ǽ����ܵĻ�ѧ����ʽΪ ��
(6)A��ͬ���칹���в����ۼ�˫ϩ(C=C=C)�ṹ��Ԫ����״������ �֣�д�����л�Ϊ�����칹��Ļ�����Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�������C11H12O3�����Ʊ�Һ�����ϵ��м���֮һ��������к���ȩ�����������������E��H��һ�������ºϳɣ�
��֪������Ϣ��
1��A�ĺ˴Ź������ױ�����ֻ��һ�ֻ�ѧ�������⣻
2��
3��������F�����ϵ�һ�ȴ���ֻ�����֣�
4��ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ���
�ش��������⣺
��1��A�Ļ�ѧ����Ϊ_________��
��2��D�Ľṹ��ʽΪ_________��
��3��E�ķ���ʽΪ___________��
��4��F����G�Ļ�ѧ����ʽΪ________,�÷�Ӧ����Ϊ__________��
��5��I�Ľṹ��ʽΪ___________��
��6��I��ͬϵ��J��I��Է�������С14��J��ͬ���칹������ͬʱ��������������
�ٱ�����ֻ������ȡ�������ڼ��ܷ���������Ӧ�����ܺͱ���NaHCO3��Һ��Ӧ�ų�CO2������______�֣������������칹����J��һ��ͬ���칹�巢��������Ӧ���ữ��˴Ź�������Ϊ����壬�ҷ������Ϊ2��2��1��д��J������ͬ���칹��Ľṹ��ʽ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
���ɱ��������ȩ�����ʺϳ�F���ϳ�·�����£�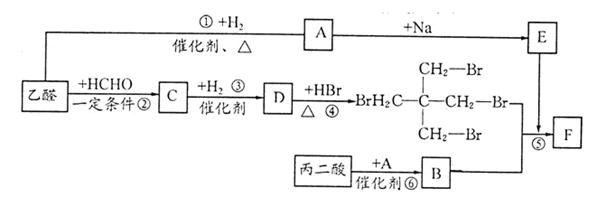

��ش��������⣺
��1��A�Ĺ����ŵ�����Ϊ ������ϳɷ��������Ʋ�C�Ľṹ��ʽ ��
��2��д����Ӧ�ܵĻ�ѧ����ʽ ����Ӧ����Ϊ ��
��3��д����������������D��ͬ���칹��Ľṹ��ʽ ��
����D������ȫ��ͬ�Ĺ�����
��ÿ��̼�����ֻ����һ��������
�ۺ˴Ź���������5�����շ�
��4����E���ڷ�̪ϡ��Һ�����Թ۲쵽�������� ����֪F���ʺɱ����ֵΪ384����E�������ºϳ�F�Ļ�ѧ��Ӧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ�ܱ������У���ӦaA��g�� bB��g����ƽ������¶Ȳ��䣬�������������һ�������ﵽ�µ�ƽ��ʱ��B��Ũ����ԭ����60%����( )
bB��g����ƽ������¶Ȳ��䣬�������������һ�������ﵽ�µ�ƽ��ʱ��B��Ũ����ԭ����60%����( )
A��ƽ��������Ӧ�����ƶ���
B������A��ת���ʼ�����
C������B��������������
D��a>b
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com