
������A�Ǻϳ���Ȼ�ĵ��壬����ʽΪC5H8��A��һϵ�з�Ӧ����(���ַ�Ӧ������ȥ)��
�ش��������⣺
(1)A�Ľṹ��ʽΪ ����ѧ������ ��
(2)B�ķ���ʽΪ ��
(3)�ڵķ�Ӧ����ʽΪ ��
(4)�ٺ͢۵ķ�Ӧ���ͷֱ��� �� ��
(5)CΪ�������������������Ǽ����ܵĻ�ѧ����ʽΪ ��
(6)A��ͬ���칹���в����ۼ�˫ϩ(C=C=C)�ṹ��Ԫ����״������ �֣�д�����л�Ϊ�����칹��Ļ�����Ľṹ��ʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��16�֣���֪B�Ǻϳɾ۶Ա��������Ҷ�������PET������Ҫԭ�ϣ�������ת����ϵ��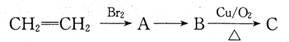
��1��B�Ĺ���������Ϊ ��
��2��A��B�ķ�Ӧ����ʽ�� ��
��3����ʢ��1ml������Һ�Ľྻ�Թ��е�������C����ˮԡ���ȿɹ۲쵽�������� ��
��4����ҵ�ϳ�PET�������£�
�� ��ϵͳ����Ϊ �������ķ�Ӧ����Ϊ ��
��ϵͳ����Ϊ �������ķ�Ӧ����Ϊ ��
���ò����õ�����Ϣ�������з���ʽ��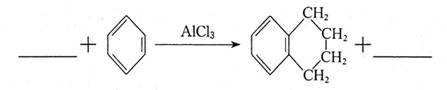
��ʵ�������ת���������Լ�Ϊ ��
��д����������������X������ͬ���칹�� ��
���������б�������������״�ṹ ������������Һ��ˮ�� ��1mol����������4molNaOH��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��֪�л���A��I֮���ת����ϵ��ͼ24��ʾ��
��A��D��B��E��I��F��Ϊͬ���칹��
�ڼ�������������Cu(OH)2����Һ�ֱ���뵽�л���I��F�У�I������������F�б�ש��ɫ
��C�����ʽ����Ȳ��ͬ������Է�������Ϊ104
��B��һ��ͬ���칹����FeC13������ɫ��Ӧ��
����������Ϣ���ش��������⣺
��1��C�к��еĹ���������Ϊ ��
��2��H�Ľṹ��ʽΪ ��
��3����Ӧ�١���������ȡ����Ӧ���� ��
��4��д��F������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ ��
��5�������Ϻ�������ȡ����������NaOH��Һ��Ӧ��������FeC13������ɫ��Ӧ��G��ͬ���칹���� �֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
2012��11��ij����ҵ�����İƱ����ܻ�������267%���Ʋ�Ʒ�е��ܻ�����ҪԴ�����ϽӾ�Ͱ��������ƹܡ���Ʒ�������ڸǵȡ��ܻ���DEHP�ĺϳ���·���£�
��1��A��ϵͳ����������Ϊ______________��C���Ӻ˴Ź���������ʾ��__________�ֲ�ͬ��ѧ��������ԭ�ӡ�C��һ�����������Ҷ�����Ӧ���ɵĸ߾�����һ����Ҫ�ĺϳ���ά���׳Ƶ��ڣ���ṹ��ʽ��__________��
��2��B���еĹ���������Ϊ__________���١��ڵķ�Ӧ���ͷֱ�Ϊ_______��_______��
��3��д����������������C��ͬ���칹��Ľṹ��ʽ:____________��
a���DZ�����λ��Ԫȡ���b����FeCl3��Һ��ʾ������ɫ��
c������̼��������Һ��Ӧ
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ�ڣ�_______________________________________________��
��Ӧ�ܣ�_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��12�֣�������������C3H6�ϳ��л��߷���E��C6H14������ͼ����ش��������⣺
��1��C6H14�˴Ź�����ֻ�����ַ壬��C6H14�Ľṹ��ʽΪ��_________________,
д��E�Ľṹ��ʽ��___________________��
��2��д��B������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ��________________________��
��3��D��ͬ���칹��ܶ࣬��������������ͬ���칹����__________��
�ٺ�̼̼˫�� ����ˮ�� ���ܷ���������Ӧ
��4����������ѧ֪ʶ����ͼ�������Ϣ�����Ҵ�Ϊ��Ҫԭ��ͨ���������ܺϳɻ�����(���Լ���ѡ����д����һ���͵�������ѧ��Ӧ�Ļ�ѧ����ʽ:
________________________________��________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����������(��ͼ��E)��һ�ֹ���������Ƽ����ܱ�ҶƬ���գ�����ֲ�����ڴ����ٶȽ���������ѿǰ���ݼ�����Ҫ���ڴ��ݡ�ij��A�ϳ�E��·�����£�A
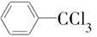
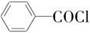
 B
B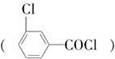
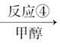
C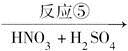 D
D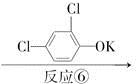
(1)д��A��C�Ľṹ��ʽ��A________��C________��
(2)д��C�й����ŵ����ƣ�________________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ��______________________________________________________��
��Ӧ��__________________________________________________________��
��Ӧ������____________________________________________________��
(4)C��ͬ���칹���ж��֡�д���˴Ź������������ֲ�ͬ��ѧ�������⣬�ҷ������Ϊ1��2��2��2�������л���Ľṹ��ʽ�� _________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
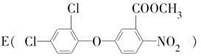
 ��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳ�
��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳ�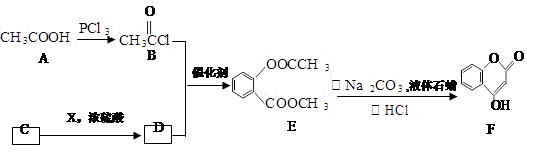
��֪��E���F�൱����E������ȥ��1��X���ӡ���ش��������⣺
��1��A��B�ķ�Ӧ������ ;
��2��E�������� �ֲ�ͬ��ѧ��������ԭ�ӣ�
��3��X������������һ�ֳ�����Ϊ��������ʣ�д����������ӵĽṹʽ ��
��4��д��D��E�Ļ�ѧ����ʽ ��
��5������������FeCl3��Һ����ɫ����C��Ϊͬ���칹����л����� �֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�����⾷ҩ���������ء���(CH3)2SO4��Ӧ��������A�����ߵĽṹ��ʽ����ͼ��
�Իش������й����⣺
��1�����������ء��ķ���ʽΪ_______��1 mol���������ء���Ũ��ˮ��Ӧʱ�������_____ mol Br2��
��2���л���A�ܷ�������ת��������G�����е�̼ԭ����һ��ֱ���ϡ�
��֪����
��R?O?CH3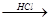 R?OH��RΪ֬������������������
R?OH��RΪ֬������������������
��C��D�Ļ�ѧ����ʽ��_________________________________________________��
��G�Ľṹ��ʽ��___ ��
��ͬʱ��������������E��ͬ���칹���� �֣�
a����FeCl3��Һ��ɫ��
b�����ܷ���ˮ�ⷴӦ���ܷ���������Ӧ��
c�������ϵ�һ��ȡ����ֻ��һ�֣���������
д����������2�ֵĽṹ��ʽ��___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������һ�ָ�Ч��������Դ��0��25mol������ȫȼ������Һ̬ˮʱ�ų�222��5KJ�������������Ȼ�ѧ����ʽ����ȷ����( )
A��2CH4(g) + 4O2(g) == 2CO2(g) + 4H2O(l)����H== +890KJ��mol��1
B�� CH4(g) + 2O2(g) == CO2(g) +2H2O(l)����H== +890KJ��mol��1
C�� CH4(g) + 2O2(g) == CO2(g) +2H2O(l)����H== -890KJ��mol��1
D�� 2CH4(g) + 4O2(g) == 2CO2(g) + 4H2O(l)����H== -890KJ��mol��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com