
�����13�֣�
��������һ����������ʧ����ҩ����ĺϳ�·�����£�
47����B��C�ķ�Ӧ����Ϊ ��
48��д��A��Ũ��ˮ��Ӧ�Ļ�ѧ����ʽ ��
49����A�Ʊ�B�Ĺ�����������������E������B��Ϊͬ���칹�壬E�Ľṹ��ʽΪ
��
50��д��ͬʱ��������������D��һ��ͬ���칹��Ľṹ��ʽ ��
������ -�����
-�����
���DZ���������ұ����ϵ�һ�ȴ���ֻ�����֣�
�۷����к����������� 51����֪��ϩ�ڴ�����������������Ӧ�������ɻ������飨 ����д����
51����֪��ϩ�ڴ�����������������Ӧ�������ɻ������飨 ����д����
�ڼ�����( )���Ҵ�Ϊԭ���Ʊ�
)���Ҵ�Ϊԭ���Ʊ�  �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���
47. ������Ӧ ��1�֣�
48. 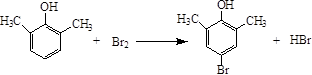 ��2�֣�
��2�֣�
49. 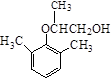 ��2�֣�
��2�֣�
50. 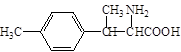 ��2�֣��𰸺��������֣�
��2�֣��𰸺��������֣�
51.��6�֣�
��˵������Na2Cr2O7����������Ҳ��ΪCu��O2��������������һ��������ֱ������Ϊ����۷֣�
�������������47.��Ӧ��ȥ�⣬Ϊ������Ӧ��48.���ǻ����ڶ�λ���巢��ȡ����Ӧ��A������λ�þ���ȡ����49.��Ӧ�ﻷ��Ϊ���Գƽṹ���ʶϻ���λ����2�֣������ֲ��50.��ͬ���칹���ж��֣���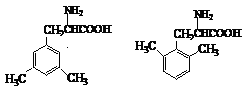 �ȣ�51.���ݲ�������ڷ�Ӧ����ǻ�����������̼ԭ�����پ������ɵá���������A��B�ķ�Ӧȷ�����ŵ����ӷ�ʽ���ٸ�����Ϣȷ�������Ҵ��Ʊ��������ѣ����̼��𰸡�
�ȣ�51.���ݲ�������ڷ�Ӧ����ǻ�����������̼ԭ�����پ������ɵá���������A��B�ķ�Ӧȷ�����ŵ����ӷ�ʽ���ٸ�����Ϣȷ�������Ҵ��Ʊ��������ѣ����̼��𰸡�
���㣺�����л���ķ�Ӧ��ϳ��й����⡣


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
A��B��C��D���Ƕ�����Ԫ�أ�A��Bͬ���ڣ� B��Cͬ�壬AԪ������������������������Bԭ���������������ڲ�������Ķ�����B��A�����ɻ�����BA2��C��A���ɻ�����CA2�� A����������D�������ӵ��Ӳ�ṹ��ͬ��������ԭ�ӵĵ��Ӳ�ṹ��ͬ��D �ĵ�����A �ĵ����ڲ�ͬ�����·�Ӧ��������D2A��D2A2����ش�
��1��д��Ԫ�ط���A�� ��B�� ��C�� ��
��2��D2A�ĵ���ʽ ��BA2�Ľṹʽ ��
��3��D2A2�Ļ�ѧʽ �������⻯�������� ɫ��
��4��C��Ԫ�����ڱ��е�λ���� ����ԭ�ӽṹʾ��ͼΪ ��
��5���Ƚ�A��B��̬�⻯��ķе� ��ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
14��)A��J�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮�����ϵ����ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬����� A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
����д���пհף�
��1��A��B��C��D��E��F��������������ͬһ��Ԫ�������ڱ���λ���� ��
��2������C��Һ�������ӷ����ǣ�д�������������ۣ�
��
��3��д����Ӧ�ٻ�ѧ����ʽ�� ��
��4��д����Ӧ�����ӷ���ʽ�� ��
��5��д����Ӧ��ѧ����ʽ�� ��
��6��д����Ӧ�������ĵ缫��Ӧʽ�� ��
��7���������仯�Ƕȿ�����Ӧ�٢ڢ��У����ڡ�H��0�ķ�Ӧ�� ������ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�����������ֿ��������쵼�����״��ֺ��Աʹ�õ�����ƿ���Ʊ�����һ���䷽�к��������������ʣ�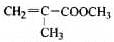 ��
��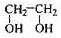 ��
��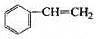 ��
��
���ף����� �����ң�������������������������������
��д���пհף�
(1)�����Լ������Ӧ����ɫ����___________�����ţ�
a. Br2/CCl4��Һ b.ʯ����Һ c.����KMnO4��Һ
(2)��ͬ���칹���ж��֣�д������һ�ֲ�����������Ľṹ��ʽ��_______
(3)����ͨ������ת�����Եõ��ң�����A��D��Ϊ�л��: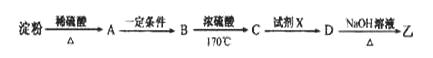
A�ķ���ʽ��___________���Լ�X������___________��
��4����֪��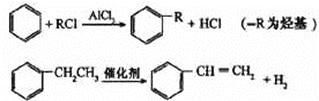
����������Ϣ���Ա�����ϩ���Ȼ���Ϊԭ�Ͼ�������Ӧ�ϳɱ�����������ȡ����Ӧ�Ļ�ѧ����ʽ�� ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��15�֣���ϩ�����ںϳ�Ӧ�ù㷺��DAP��֬���塣���з�Ӧ�����еķ�Ӧ����������������ʡ�ԡ�
��֪�� RCOOR����R����OH��RCOOR������R��OH��R��R����R�������������� 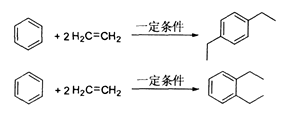
��1��DAP��֬����ķ���ʽΪ ��
��2��B�����������ŵ������� ��B��C�ķ�Ӧ���� ��
��3��E����Է�������Ϊ162�� 1H�˴Ź�����3���źš�д��E�Ľṹ��ʽ ��
��4����д����Ӧ�ڵĻ�ѧ����ʽ ��
��5��D�ж���ͬ���칹�壬��д����������������ͬ���칹��Ľṹ��ʽ ��
A������NaOH��Һ��Ӧ
B��������ֻ��������ͬ��ȡ�������ұ����ϵ�һ�ȴ���������
��6���Ա�ϩΪԭ�ϣ��������Լ���ѡ��д���Ʊ��������͵ĺ�������ͼ��ע���Լ���
������ע���ϳ�·�ߵ���д��ʽ��������ʵ������ͼ��
CH3CHO CH3COOH
CH3COOH CH3COOCH2CH3
CH3COOCH2CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��16�֣��л���F�Ǻϳ�ҩ����м��塣��������ѧ��ѧ���л�����кϳɣ��䷽�����£�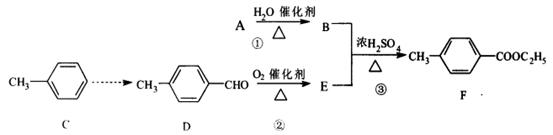
A�IJ�����־��һ������ʯ�ͻ�����չˮƽ��
��1�� D�ķ���ʽ�� ��1mol D��ȫȼ�������� mol O2
��2����Ӧ�ٵĻ�ѧ��Ӧ����ʽ�� ���÷�Ӧ�ķ�Ӧ������ ����ʳƷ��ҵ��E�������ζ������� ����
��3��д����Ӧ�۵Ļ�ѧ��Ӧ����ʽ�� ��
��4��д����������������E��ͬ���칹�壺 ����ֻ������һ�ּ��ɣ�
�����б��������ܹ�����������Ӧ�����ܷ���ˮ�ⷴӦ��ˮ�������FeCl 3��Һ����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��15�֣�������F�Ǻϳ�������Ѫ�ܲ�����Ҫҩ��������İ����м��塣����һ�ֺϳ�·�����£�
�ش��������⣺
��1��A�Ļ�ѧ����Ϊ �� ��2�� B����C�ķ�Ӧ����Ϊ ��
��3����C����D�Ļ�ѧ����ʽΪ�� ����4��E�ķ���ʽΪ ��
��5��F�Ľṹ��ʽΪ ��1mol F�����������Ʒ�Ӧ�����������ڱ�״���µ����Ϊ ��
��6��B�ж���ͬ���칹�壬д��ͬʱ��������������B������ͬ���칹��Ľṹ��ʽ ��
�ټ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ��
�ں˴Ź�������ͼ��4�����շ塢�ҷ����֮��Ϊ1��2��6��3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�л��������������洦�ɼ�������ǿ����ҩ������ʵ�ij�ֺϳ�·�����£� 
��ش��������⣺
��1�������ķ���ʽ�ǣ� ��
��2������˵����ȷ���� ������ţ���
a����Ӧ����������Ӧ��
b������������ϩȩ��A��B����������Ʒ�Ӧ�ų�������
c������A��B��E��������ˮ�����ӳɷ�Ӧ����ɫ��
d���л��������Է���ˮ�ⷴӦ��
��3������B���� ������ţ���
a������ b��ϩ�� c���� d���� e�� ��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
��5����Ӧ�IJ���Ϊ������FeCl2�� ���ڴ��������£�����������ӳɺ�IJ���Ϊ��д�ṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��27�֣����ĺϳ�ԭ��Ϊ��N2��g��+3H2��g��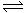 2NH3��g������H= ��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺
2NH3��g������H= ��92��4 KJ��mol��1������500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯����ͼ���ش��������⣺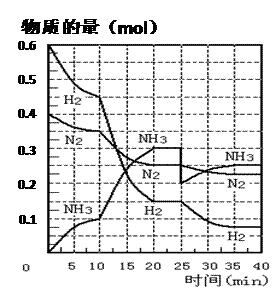
��1��10 min����NH3��ʾ��ƽ����Ӧ���� ��
��2����10 ~20 min�ڣ�NH3Ũ�ȱ仯��ԭ������� ��
| A�����˴��� | B����С������� |
| C�������¶� | D������NH3���ʵ��� |
 2NH3��g�� +
2NH3��g�� + 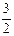 O2��g�� ����H =" a" kJ��mol��1
O2��g�� ����H =" a" kJ��mol��1| T/K | 303 | 313 | 323 |
| NH3������/��10-6mol�� | 4��8 | 5��9 | 6��0 |
 2NH3��g�� ��H= ��92 ��4kJ��mol��1
2NH3��g�� ��H= ��92 ��4kJ��mol��1�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com