���
�⣺����ͼ���֪��0-2 min�ڣ����������ʵ���������0.1mol�����Ȼ����HI��0.2mol��ʹ��ƽ����Ӧ����v��HI��=
=0.1 mol/��L?min����ƽ��ʱ�����͵��Ũ�ȶ���0.1mol/L�����⻯�����0.8mol/L��ʹ�ø��¶���ƽ�ⳣ��K=
| 0.1mol/L��0.1mol/L |
| (0.8mol/L)2 |
�������淴Ӧ��ƽ�ⳣ����64��ƽ�ⳣ��ֻ���¶��й�ϵ��ѡ��a����ȷ�����ڷ�Ӧǰ��������䣬���Ը÷�Ӧ�ǵ�Ч�ģ����ѡ��b��ȷ��d����ȷ��Ũ�����ӣ���Ӧʱ�����ﵽƽ���ʱ����٣�ѡ��c����ȷ����ѡb��
�ʴ�Ϊ��0.1mol/��L?min���� 64��b��
��pH=3����������ʵ���Ũ��=1��10
-3 mol/L��
���а�ˮ�����ʵ���Ũ����1��10
-3 mol/L����һˮ�ϰ���������ʣ�ֻ�в��ֵ��룬���Ԣ��а�ˮ��Ũ�ȴ���1��10
-3 mol/L���������������ӵ�Ũ����1��10
-3 mol/L��
�����������������ǿ����ʣ������Ӻ������������к�ʱ��1��1�Ĺ�ϵ�������Ӻ����������ӵ�Ũ����ȣ�����a��d�������ȣ���a=d��
�ڵİ�ˮŨ�ȴ��ڢٵ�Ũ�ȣ��к���ͬ���ʵ��������ᣬ��ˮ��Ũ��Խ��ʹ�õİ�ˮ�����ԽС������c��b������Ͱ�ˮ��Ӧ���ɵ��Ȼ����ǿ�������Σ�ˮ���ʹ��Һ�����ԣ�Ҫ��ʹ��Һ�����ԣ���ˮ�����ʵ���Ӧ��������Ĵ�Щ��������Ũ�ȺͰ�ˮ��Ũ�����ʱ����ˮ�����bӦ������������a������Һ�����a��b��
�ڢ������������ӵ�Ũ����ȣ�һˮ�ϰ���һԪ������ʣ�����������ǿ����ʣ�����ˮ��Ũ�ȴ��ڢ�������������Ũ�ȣ��к���ͬ���ʵ�����������ʱ�������õİ�ˮ�����С�ڢ�����������Һ���������c��d=a��
����a��b��c��d�Ĺ�ϵb��a=d��c��
�ʴ�Ϊ��b��a=d��c��
��1�������ж�ƽ��״̬�ķ�����V
��=V
���������ֵ�Ũ�ȱ��ֲ�����˵���Ѵ�ƽ�⣬
A��Cr
2O
72-��CrO
42-��Ũ����ͬȡ������ʼŨ�Ⱥ�ת���������ж�ƽ�⣬��A����
B��2v��Cr
2O
72-��=v��CrO
42-���������ж����淴Ӧ���ʹ�ϵ�������ж�ƽ�⣬��B����
C����Һ��pHֵ���ֲ��䣬˵��������Ũ�Ȳ��䣬���ж�ƽ�⣬��C��ȷ��
D����Һ����ɫ���䣬Ϊ�����������ж�ƽ�⣬��D��ȷ��
�ʴ�Ϊ��CD��
��2���ữʱ�����ķ�ӦΪ��2CrO
42-+2H
+?Cr
2O
72-+H
2O����1L�ữ��������Һ�к���Ԫ�ص�����Ϊ28.6g��CrO
42-��
ת��ΪCr
2O
72-��˵����Ԫ��
ת��ΪCr
2O
72-�����غ��й�ϵʽ��2Cr��2CrO
42-��Cr
2O
72- 2 1
n��Cr
2O
72-��
��n��Cr
2O
72-��=0.25mol��n��CrO
42-��
ʣ��=0.05mol��
����ữ��������Һ��c��Cr
2O
72-��=
=0.25mol?L
-1�� c��CrO
42-��
ʣ��=0.05mol?L
-1����H
+�����ʵ���Ũ��Ϊamol/L��
2CrO
42-+2H
+�T?Cr
2O
72-+H
2O
ƽ�⣨mol/L�� 0.05 a 0.25
ƽ�ⳣ��K=
�T10
14��
��a=1.0��10
-6mol��PH=6��
�ʴ�Ϊ��0.25mol/L��6��
��3��������K
sp[Cr��OH��
3]=1��10
-32��Ҫʹ�������ˮ��c��Cr
3+������1��10
-5mol/L����c��Cr
3+����c
3��OH
-��=1��10
-32��c��OH
-��=1��10
-9mol/L��pH=5��
�ʴ�Ϊ��5��

 ��ѧѧ���еĻ�ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ���������������ԭ������ش��������⣺
��ѧѧ���еĻ�ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ���������������ԭ������ش��������⣺



 ���ķ�ˮ�������������س�ȥ����ԭ����ͼ��ʾ��������
���ķ�ˮ�������������س�ȥ����ԭ����ͼ��ʾ��������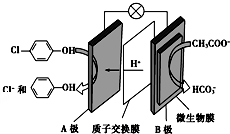
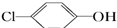 +2e-+H+
+2e-+H+ +Cl-
+Cl-