
����Ŀ���״���һ����Ҫ���л�����ԭ�ϡ�
��1����֪��
��C2H4(g)+H2O(g)��C2H5OH(g) ��H1=-45.5 kJ/mol
��2CH3OH(g)��CH3OCH3(g)+H2O(g) ��H2=-23.9 kJ/mol
��C2H5OH(g)��CH3OCH3(g) ��H3=+50.7 kJ/mol
��д����ϩ��ˮ�����������ɼ״�������Ȼ�ѧ����ʽ��__________��
��2���ϳɼ״��ķ�ӦΪ��CO(g)+2H2(g) ![]() CH3OH(g) ��H����ͬ�����£����ݻ���ͬ��a��b��c��d��e����ܱ������зֱ������������ʵ���֮��Ϊ1:2��CO��H2�Ļ�����壬�ı��¶Ƚ���ʵ�飬��÷�Ӧ���е�t minʱ�״������������ͼ����ʾ��
CH3OH(g) ��H����ͬ�����£����ݻ���ͬ��a��b��c��d��e����ܱ������зֱ������������ʵ���֮��Ϊ1:2��CO��H2�Ļ�����壬�ı��¶Ƚ���ʵ�飬��÷�Ӧ���е�t minʱ�״������������ͼ����ʾ��
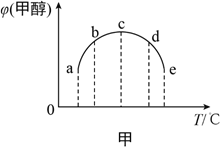
���¶����״���������������ԭ����__________��
�ڸ���ͼ���жϦ�H__________���>������<����=����0��
��3��Ϊ���о��״�ת��Ϊ�����ѵķ�Ӧ������ij�о���С�������������Ϊ1.0 L�ĺ����ܱ������з�����Ӧ��2CH3OH(g) ![]() CH3OCH3(g)+H2O(g) ��H2=-23.9 kJ/mol��
CH3OCH3(g)+H2O(g) ��H2=-23.9 kJ/mol��
������� | �¶�/�� | ��ʼ���ʵ���/mol | ƽ�����ʵ���/mol | |
CH3OH(g) | CH3OCH3(g) | H2O(g) | ||
�� | T1 | 0.20 | 0.080 | 0.080 |
�� | T1 | 0.40 | A | a |
�� | T2 | 0.20 | 0.090 | 0.090 |
��T1�¶��¸÷�Ӧ��ƽ�ⳣ��K=__________����Ӧ�¶�T1__________T2������ڡ���С�ڡ�����
����������a=__________��
������˵����˵����Ӧ�ﵽƽ��״̬����__________������ĸ����
A������������ѹǿ���ٱ仯
B����CH3OH��CH3OCH3��ʾ�ķ�Ӧ����֮��Ϊ2:1
C�����������ܶȲ���
D��������CH3OH��CH3OCH3��Ũ��֮��Ϊ2:1
E�����������c(CH3OCH3)����
���𰸡� C2H4(g)+2H2O(g)��2CH3OH(g) ��H=+29.1 kJ/mol a��c��Ӧδ��ƽ�⣬�¶�Խ�߷�Ӧ����Խ�죬�״����������Խ�� < 4 ���� 0.16 E
����������1�����ݸ�˹���ɢ�-��+�ۿɵ�C2H4(g)+2H2O(g) ![]() 2CH3OH(g)����H=��H1-��H2+��H3=+29.1 kJ/mol���ʴ�Ϊ��C2H4(g)+2H2O(g)
2CH3OH(g)����H=��H1-��H2+��H3=+29.1 kJ/mol���ʴ�Ϊ��C2H4(g)+2H2O(g) ![]() 2CH3OH(g) ��H=+29.1kJ/mol��
2CH3OH(g) ��H=+29.1kJ/mol��
��2����a��b��û�дﵽƽ����c��ǡ��ƽ�⣬d��eΪƽ��״̬��a��c֮��ĵ������ƽ��״̬���¶����ߣ���Ӧ���ʼӿ죬��ͬʱ���ڣ����ɵļ״����࣬�״��������������
�ʴ�Ϊ��a��c��Ӧδ��ƽ�⣬�¶�Խ�߷�Ӧ����Խ�죬�״����������Խ��
��c��e֮��ĵ��Ϊƽ��״̬���¶����ߣ�ƽ�������ȷ����ƶ����÷�Ӧ���¶��������״������������С����ƽ�����淴Ӧ�����ƶ�����������ӦΪ���ȷ�Ӧ����H��0��
�ʴ�Ϊ������
��3�������ݱ������ݣ��г�����I�з�Ӧ����ʽ��
2CH3OH(g) ![]() CH3OCH3(g)+H2O(g)
CH3OCH3(g)+H2O(g)
��ʼ���ʵ���/mol 0.20 0 0
�仯���ʵ���/mol 0.16 0.080 0.080
ƽ�����ʵ���/mol 0.04 0.080 0.080
�������Ϊ1L����ƽ�ⳣ��K=![]() =
=![]() =4��
=4��
�÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ���CH3OCH3(g)������С������I������������ʼʱͶ���CH3OH���ʵ�����ͬ����ƽ��ʱ����CH3OCH3(g)�����ͣ�˵��������ķ�Ӧ�¶Ƚϸ�����T1����T2��
�ʴ�Ϊ��4�����ڣ�
����Ӧ2CH3OH(g) ![]() CH3OCH3(g)+H2O(g)�Ƿ�Ӧǰ�������������ķ�Ӧ�����º���������
CH3OCH3(g)+H2O(g)�Ƿ�Ӧǰ�������������ķ�Ӧ�����º���������
���������ڵ�Чƽ���������ʼ���ʵ�����I��2������ƽ��ʱ����������ҲΪ���2������a=0.08��2=0.16��
�ʴ�Ϊ��0.16��
�۵��������ٸı�ʱ����Ӧ�ﵽƽ��״̬��A���÷�ӦΪ��Ӧǰ��������Ŀ���䣬�����������ʵ���n�Ƕ���������pV=nRT��֪�����º���ʱ��p��n�����ȣ�����������ѹǿΪ��������ѹǿ���ٱ仯����˵����Ӧ�ﵽƽ��״̬����A����B������Ӧ�У�CH3OH��CH3OCH3�ķ�Ӧ����֮��һֱΪ2:1���淴Ӧ�У�CH3OH��CH3OCH3�ķ�Ӧ����֮��ҲһֱΪ2:1������CH3OH��CH3OCH3������֮��Ϊ2:1����˵����Ӧ�ﵽƽ��״̬����B����C����=m/V��������������Ƕ���������ʱ���Ҳ�Ƕ��������ܶ�Ϊ�������ܶȲ��䲻��˵����Ӧ�ﵽƽ��״̬����C����D��ƽ��ʱCH3OH��CH3OCH3��Ũ��֮�ȿ���Ϊ2:1��Ҳ���ܲ���2:1������˵����Ӧ�ﵽƽ��״̬����D����E��c(CH3OCH3)�DZ�������c(CH3OCH3)���䣬˵����Ӧ�ﵽƽ��״̬����E��ȷ��
�ʴ�Ϊ��E��


 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. 2.8 g�����麬���ۼ���ĿΪ0.4NA
B. ����������ˮ��Ӧ������0.1 mol����ʱ��ת�Ƶĵ�����Ϊ0.4NA
C. 142 g Na2SO4��Na2HPO4���������У�������������Ϊ3NA
D. �ö��Ե缫���CuSO4��Һ���������0.1 molCu(OH)2��ʹ��Һ��ԭ�����·��ת�Ƶ�����Ϊ0.2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��N2O5��һ����������������һ���¶��¿ɷ������з�Ӧ��2N2O5(g) ![]() 4NO2(g)+O2(g)����H>0��T1�¶��µIJ���ʵ������Ϊ��
4NO2(g)+O2(g)����H>0��T1�¶��µIJ���ʵ������Ϊ��
t/s | 0 | 500 | 1000 | 1500 |
c(N2O5)/(mol��L-1) | 5.00 | 3.52 | 2.50 | 2.50 |
����˵������ȷ���ǣ� ��
A. 500 s��N2O5�ֽ�����Ϊ2.96��10-3 mol��L-1��s-1
B. �����������䣬T2�¶��·�Ӧ��1000sʱ���N2O5(g)Ũ��Ϊ2.98 mol��L-1����T1<T2
C. T1�¶��µ�ƽ�ⳣ��ΪK1=125��1000sʱת����Ϊ50%
D. T1�¶��µ�ƽ�ⳣ��ΪK1��T3�¶��µ�ƽ�ⳣ��ΪK3����K1>K3����T1>T3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݺ�ˮ�ۺ����õĹ�ҵ����ͼ,�ж�����˵������ȷ������ ��

A. ��ȥ����������(Mg2+��SO42-��Ca2+),�����ҩƷ˳��Ϊ��NaOH��Һ��BaCl2��Һ��Na2CO3��Һ�����˺������
B. �ڹ������н�MgCl2��6H2O���ռ����Ƶ���ˮMgCl2
C. ������ת���Ƕȿ�,�ȼҵ��ⱥ��ʳ��ˮ��һ��������ת��Ϊ��ѧ�ܵĹ���
D. �ӵ���������������Ŀ���Ǹ���Br2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������(V2O5,Ħ������Ϊ182g��mol-1)������ѧ��ҵ�еĴ������㷺����ұ�𡢻�������ҵ��V2O5��һ�ֳȻ�ɫƬ״����,����ˮ���������Ҵ�,����ǿ������,�������������ij�о�С�齫��ij�ַ�(��Ҫ����V2O5,��������Al2O3��Fe2O3)����ȡV2O5��ʵ�鷽��������£�

��֪��NH4VO3�ǰ�ɫ��ĩ,������ˮ,��������ˮ,�������Ҵ����ѡ�
2NH4VO3![]() V2O5+2NH3��+H2O
V2O5+2NH3��+H2O
��ش�
��1��������������ʵ��װ������ͼ��ʾ,���߿�����Ϊ���ʵ�������________��(����)
![]()
![]()
![]()
![]()

��2������pHΪ8��8.5��Ŀ��________��
��3��������ϴ�Ӳ���ʱ,��ѡ�õ�ϴ�Ӽ�_________��(����)
A����ˮ B����ˮ C���Ҵ� D��1%NH4Cl��Һ
��4������������ʱ,�����������������յĿ���ԭ��________��
��5�����Ṥҵ��,SO2ת��ΪSO3�Ĵ�����ѡ��V2O5,�����̾��������,���䲹��������________(�û�ѧ����ʽ��ʾ),4VO2+O2=2V2O5��
��6����0.253g��Ʒ����ǿ����Һ��,�������,����pHΪ8��8.5,��Ӧ�����Һ�м��������ữ��KI��Һ(����),��Һ�к���V3+,�μ�ָʾ��,��0.250mol��L-1Na2S2O3��Һ�ζ�,�ﵽ�յ�����Na2S2O3����Һ20.00mL,��ò�Ʒ�Ĵ���Ϊ________��(��֪��I2+2Na2S2O3=Na2S4O6+2NaI)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij��ɫ��Һ�У����ܺ���Mg2����Al3����Fe2����NH4����H����AlO2����Cl���е�һ�ֻ������ӣ������л����ص���NaOH��Һֱ���������������������ʵ���������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ���ɴ�ȷ������ɫ��Һ�к��е�������( )

A. Mg2����Al3����Fe2����Cl��
B. H����Mg2����Al3����NH4��
C. H����Mg2����Al3����NH4����Cl��
D. Mg2����NH4����AlO2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������þ����������������ݼ��ȣ�ʵ�����Ʊ�Mg(C1O3)2��6H2O ����������:
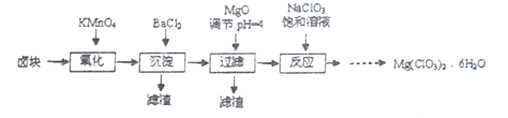
��֪����±����Ҫ�ɷ�ΪMgCl2��6H2O������MgSO4��FeCl2�����ʡ�
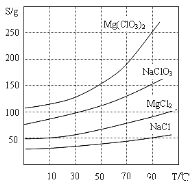
�����ֻ�������ܽ��(S)���¶�(T)�仯������ͼ��ʾ��
��1����������Ҫ����Ҫ����������_________��
��2����MgO�����������������Ҫ�ɷֵĻ�ѧʽΪ_________��
��3������NaClO3������Һ������Ӧ�Ļ�ѧ����ʽΪ__________���ٽ�һ����ȡMg(ClO3)2��6H2O��ʵ�鲽������Ϊ����________��______��ϴ�ӣ��ڽ���Һ��ȴ�ᾧ���۹��ˡ�ϴ����
��4����Ʒ��Mg(ClO3)2��6H2O�����IJⶨ������֪Mg(ClO3)2��6H2O��Ħ������Ϊ299 g/mol��
����1��ȷ����3.50g��Ʒ���100 mL��Һ��
����2��ȡ10.00mL����ƿ�У�����10.00mLϡ�����20.00mL1.000mol��L��1��FeSO4��Һ���ȡ�
����3����ȴ�����£���0.100mol/LK2Cr2O7 ��Һ�ζ�ʣ���Fe2+���յ㡣��Ӧ�ķ���ʽΪ��Cr2O72����6Fe2+��14H+��2Cr3+��6Fe3+��7H2O��
����4��������2��3�ظ����Σ�ƽ������K2Cr2O7 ��Һ15.00mL��
�� д������2�з�����Ӧ�����ӷ���ʽ__________��
�� ��Ʒ��Mg(ClO3)2��6H2O����������Ϊ_______����������С�����һλ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�����������������Ϣ��ϵ���У�����˵������ȷ����
A. ��¯ˮ���к��е�CaSO4��������Na2CO3��Һ�������������ȥ
B. ��һ���·��ֵĹ�̬̼������̼�Ľṹ��Ϊ��������ĭ�����������ƺ��࣬�ܶ�С���д��ԡ�����̼����ʯ�Ĺ�ϵ�ǻ�Ϊͬ��������
C. ����Ȳ��I2��Na �Ȳ��Ӵ�������γ�һ�ֵ������ϣ��õ��������й̶����ۡ��е�
D. ��ҵ�ϵ�����ڵ��Ȼ��ơ��Ȼ�þ������������ȡ�ơ�þ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ȷ£�CHCl3��������ˮ������һ��������ˮ�����������ᣬ����һ���Ǽ��ᣨHCOOH������19�����ȷ¹㷺�������������ɡ���ȩƯ�۷����Ƶá��ڹ��������£��ȷ��ױ������������ɾ綾������COCl2����һ�ֻ��������ȷ���ҪС�ڸֹ��������䣬ʹ��ǰҪ�������Ƿ���ʡ�
��1��CHCl3�ĵ���ʽΪ__________��HCOOH��C���ӻ���ʽΪ__________��
��2��Ư��������Ԫ�صļ����ӵİ뾶�Ĵ�С˳��Ϊ__________�������ӷ��ű�ʾ����
��3���ȷ²�����ˮ������������ˮ��ԭ����__________��
��4��Fe2+����Χ�����Ų�ͼΪ__________��
��5��������һ��ͬ��������ľ���Ϊ���������������þ�����ԭ�ӵ���λ��Ϊ__________������ԭ�ӵİ뾶Ϊa cm����þ�����ܶ�Ϊ__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com