
��������������ͭ�Ľᾧˮ����仯ѧʽΪCuSO4?5H2O���ڼ�������£����¶Ȳ�ͬ���������������һϵ�еı仯���õ���ͬ��ɵĹ��塣
��1����ȡ0.1000 g�������ʵĵ�����������ƿ�У�����0.1000 mol/L����������Һ28.00 mL����Ӧ��ȫ����������������0.1000 mol/L����ζ����յ㣬��������10.08 mL���������е�������������Ϊ___________��
����֪��CuSO4 + 2NaOH �� Cu(OH)2 + Na2SO4�������е����ʲ����ᡢ�Ӧ��
��2����1.250 g�����ĵ����������������м���һ��ʱ�䣬���ʣ���������Ϊ0.960 g��ʣ������нᾧˮ����������Ϊ__________(������λС��)��
��3������ˮ����ͭ������650�����ϣ��ɵõ���ɫ������ͭ����������������������Ļ�����塣�ֽ�9.600 g��ˮ����ͭ��ּ��ȷֽ�Ϊ����ͭ�������ɵ�����ͨ�����������ռ�����ʯ�ң������ռ�����4.416 g�������������ռ������������������ε����ʵ���֮�ȡ�
��4����ˮ����ͭ���ȷֽ������֮ͭǰ����һ�ֻ�ɫ�м����X���֣��仯ѧʽ���Ա�ʾΪCuaOb(SO4)c��a��b��cΪ����������X����ˮ�У��в��ܵ���ɫ����Y���ɣ���ѧʽΪCuSO4��nCu(OH)2����ͬʱ����2/3�����������ˮ������Y���м�����ˮ����ʧȥ11.9%����������֪X��Y��������ϡ���ᡣͨ������ȷ��X��Y�Ļ�ѧʽ��
��1��0.98��3�֣���
��2��0.167��3�֣���
��3��3:2��4�֣���
��4��X��Cu2OSO4��2�֣���Y��CuSO4��3Cu(OH)2��2�֣�
������̣�
�ȼ���Y�Ļ�ѧʽ��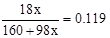 �����x=3
�����x=3
��ΪCuSO4��3Cu(OH)2
��2/3���������ˮ����Y������ϡ�����֪�ܽ�ijɷ�ΪCuSO4�������ʵ���Ϊ������2�����ɴ˿�֪��ɫ�м���ﺬ��Cu2+��SO42-�ĸ�����Ϊ2:1�����ݵ���غ��֪�仯ѧʽΪCu2OSO4��
���������������1��n(NaOH)="0.1000" mol/L*0.028mL=2.8*10-3mol��n(H2SO4 )="0.1000" mol/L*0.0108 L=1.08*10-3mol���͵�����Ӧ�������������ʵ���Ϊ��2.8*10-3mol-2.16*10-3mol=6.4*10-4mol���������ʵ���Ϊ3.2*10-4mol������Ϊ3.2*10-4*250g��������������Ϊ3.2*10-4*250g/0.1g=0.8��
��2��1.250 g �����ĵ�������������ͭ������Ϊ1.25*160/250=0.8g��ʣ���������Ϊ0.960 g��ˮ������Ϊ0.16g��ʣ������нᾧˮ����������Ϊ0.16/0.96=0.167��
��3��9.600 g��ˮ����ͭ�����ʵ���Ϊ0.06mol�����ռ�����4.416 g���������ɵĶ���������������������֮�͡�����������������������ʵ����ֱ�Ϊx,y������x+y=0.06�� 64x+80y=4.416�����y=0.036,x=0.024���������ʵ���֮��Ϊ3��2��
��4���ȼ���Y�Ļ�ѧʽ��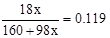 �����x=3 ��ΪCuSO4��3Cu(OH)2����2/3���������ˮ����Y������ϡ�����֪�ܽ�ijɷ�ΪCuSO4�������ʵ���Ϊ������2�����ɴ˿�֪��ɫ�м���ﺬ��Cu2+��SO42-�ĸ�����Ϊ2:1�����ݵ���غ��֪�仯ѧʽΪCu2OSO4��
�����x=3 ��ΪCuSO4��3Cu(OH)2����2/3���������ˮ����Y������ϡ�����֪�ܽ�ijɷ�ΪCuSO4�������ʵ���Ϊ������2�����ɴ˿�֪��ɫ�м���ﺬ��Cu2+��SO42-�ĸ�����Ϊ2:1�����ݵ���غ��֪�仯ѧʽΪCu2OSO4��
���㣺���⿼��������ͨ������Ի�ѧʽ��ȷ����������ɵļ��������


 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ���������С��.
(1��0.5 mol H2O������Ϊ g������____________������,___________�����ӡ�
(2) 0.01molij���ʵ�����Ϊ1.08g��������ʵ�Ħ������Ϊ__________________��
��3������50 mL 0.2 mol/L CuSO4��Һ����ҪCuSO4_____________g����ҪCuSO4��5H2O _____g��
��4����ͼ����У��ѧʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݡ�
�ٸ�Ũ��������ʵ���Ũ��_____________��
���ø�Ũ��������200mL1mol/L��ϡ���ᣬ��Ͳ������ȡ��Ũ����������_________mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����֪10g CaCO3�ֽ���Ҫ����17.56kJ ��������д��̼��Ʒֽ���Ȼ�ѧ��Ӧ����ʽ�� ��
��2������5.00 g̼���ƺ�̼�����ƵĻ�����Ӧ��ɺ����������������0.31 g����ԭ�������̼���Ƶ�����Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�±����ó������ⶨKHCO3��Na2CO3��������ɵ�������ݡ�ʵ�������ÿ�γ�ȡһ����������Ʒ����ˮ�Ƴ���Һ,�����еμ���ͬŨ�ȵ�Ba(OH)2��Һ,ÿ��ʵ�����ַ�Ӧ��ʵ���¼���±�:
| ʵ����� | ��ȡ��Ʒ�� ����/g | ����Ba(OH)2 ��Һ�����/L | ������ɳ��� ������/g |
| 1 | 0.518 | 0.5 | 0.985 |
| 2 | 1.036 | 0.5 | |
| 3 | 1.554 | 0.5 | 2.955 |
| 4 | 2.072 | 0.5 | 3.940 |
| 5 | 2.590 | 0.5 | 3.940 |
| 6 | 3.108 | 0.5 | 3.940 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
���ð������������ᣬ�̶���ȡ����李������������������������Ϊ90%����ת��Ϊ����淋�ת����Ϊ94%����100 t�������������ٶ�����泥�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��10mLNa2CO3��Na2SO4�Ļ����Һ�м����������Ȼ�����Һ�����ɳ���������Ϊ6.27g�������ó����м�������ϡ���ᣬ�����������ٵ�2.33g�����ų����壬�Լ��㣺
��1��ԭ�������Na2SO4�����ʵ���Ũ�ȣ�
��2���ڱ�״���²�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
������CO2����ͨ��7.8gNa2O2���塣��ش�
��1���ڱ�״���£��������������Ϊ����L��
��2����������Ӧ��Ĺ������Ƴ�500 mL��Һ��������Һ���ʵ���Ũ���Ƕ���mol/L��
��3������������Һʱ�����²����ᵼ����������ҺŨ��ƫ�͵���__________��
��ת����Һ��û��ϴ���ձ��Ͳ���������������Һʱ����ƿ��������ˮ��
���ڶ���ʱ�����ӿ̶��ߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��117 g NaCl����ˮ���Ƴ�1 L��Һ���ش��������⣺
��1������Һ��NaCl�����ʵ���Ũ��Ϊ���� ��
��2������1mol��L��1��NaCl��Һ500mL����Ҫ����Һ�����Ϊ���٣�
��3����2�������Ƶ���Һ����ͨ��һ������HCl�������Һ��Cl�������ʵ���Ũ��Ϊ3 mol��L�� 1(������Һ�������)������Һ��H�������ʵ���Ũ��Ϊ���٣�ͨ��HCl��������(��״����)Ϊ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�Ի�����(FeS2)�������ƺ�������Һ��Ϸ�Ӧ�Ʊ������������壬����ˮ���ջ�ö���������Һ���ڴ˹�������Ҫ�������˵��¶ȣ����¶Ȳ���������Ӧ���ӣ�Ӱ������ClO2����Ĵ��ȣ��һ�Ӱ��ClO2����������ʡ����������ͼ6��ʾ����ش���������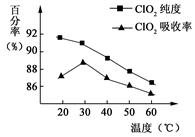
��1����ͼ��֪����Ӧʱ��Ҫ���Ƶ������¶��������棬�ﵽ��Ҫ���ȡ�����˴�ʩ��������
��2����֪���������е���Ԫ�������������±�ClO3��������SO42����д���Ʊ��������ȵ����ӷ���ʽ��������
��3��ijУ��ѧѧϰС�����ԡ�m(ClO2)/m(NaClO3)����Ϊ����ClO2���ʵ�ָ�ꡣ��ȡNaClO3��Ʒ����6.0g��ͨ����Ӧ�����տɵ�400 mL ClO2��Һ��ȡ��20 mL������37.00 mL 0.500mol��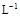 (NH4)2Fe(SO4)2 ��Һ��ַ�Ӧ������Fe2+����0.0500 mol��
(NH4)2Fe(SO4)2 ��Һ��ַ�Ӧ������Fe2+����0.0500 mol��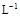 K2Cr2O7����Һ�ζ����յ㣬����20.00 mL����Ӧԭ�����£�
K2Cr2O7����Һ�ζ����յ㣬����20.00 mL����Ӧԭ�����£�
4H++ClO2+5Fe2+=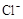 +5Fe3+ +2H2O
+5Fe3+ +2H2O
14H+ +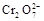 +6 Fe2+ =2Cr3+ + 6 Fe3+ +7H2O
+6 Fe2+ =2Cr3+ + 6 Fe3+ +7H2O
�Լ���ClO2�ġ����ʡ�����д��������̣�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com