
���ȸʯ��һ�ֺ�ͭ�Ŀ�ʯ����ͭ��̬Ϊ
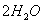 ��ͬʱ����
��ͬʱ���� �����ʡ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��
�����ʡ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��
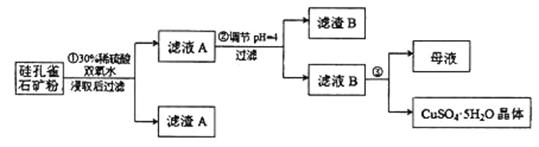
��ش��������⣺
����ɲ������ϡ������ ������Ӧ�Ļ�ѧ����ʽ
������Ӧ�Ļ�ѧ����ʽ
 ��
��
�����ӷ���ʽ��ʾ˫��ˮ������_____________________________��
�Ʋ���ڵ�����ҺpHѡ�õ�����Լ���__________________
A. B.CuO C.A12O3 D.
B.CuO C.A12O3 D.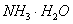
���й��������↑ʼ��������ȫ������pH���±���
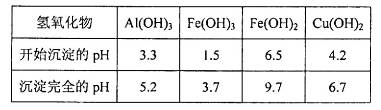
���ϱ���֪������ҺpH=4ʱ��������ȫ��ȥ��������______��������ȫ��ȥ��������________��
����ҺBͨ������Ũ���������Ũ��Ϊԭ����һ�룩����ȴ�ᾧ���Եõ� ���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�
���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�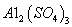 ������Һ��
������Һ��
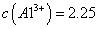 mol
mol ______________��
______________��
����Ҫ�ⶨ����ͭ�� ���нᾧˮ�ĺ�������Ҫ�������Ǿƾ��ơ�������ƽ�����Ǽܡ������ǡ���������������������ǯ���в���ҩ�ס�_________________��ʵ�����������ͭ�������ʧˮ���ڿ�����ȴ���������ⶨ���______________(�ƫ�ߡ��� ��ƫ�͡����䡱)��
���нᾧˮ�ĺ�������Ҫ�������Ǿƾ��ơ�������ƽ�����Ǽܡ������ǡ���������������������ǯ���в���ҩ�ס�_________________��ʵ�����������ͭ�������ʧˮ���ڿ�����ȴ���������ⶨ���______________(�ƫ�ߡ��� ��ƫ�͡����䡱)��
 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й��������ʵ�Ӧ����ȷ����
A���л���ũҩ��Ϊ����������������������ʣ�����ˮ�ȼ�������������ˮ����ⶾ
B����������������̫���ܵ�ذ����Ҫ����
C����������ͨ������KMnO4��Һ����Һ�Ϻ�ɫ��ȥ��������SO2��Ư����
D��������ŨH2SO4��ŨHNO3����ʹCu�����ۻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ǹ������Դ���⣬���ú�ˮ����ȡ�������Ʒ��
��1���ȼҵ���Ե��ʳ��ˮΪ������ѧ��ҵ��д�����ʳ��ˮ�Ļ�ѧ����ʽ_________
__________________________________________________________.
��2���Ӻ�ˮ����ȡ�峣�ô���������������(Cl2)����ˮ���廯�ƣ�NaBr���е����û����������ÿ���������(Br2)������ʾ��ͼ���£�

��д��������NaBr���û����嵥�ʵĻ�ѧ����ʽ��____________________;�÷�Ӧ��pH=3�����������½��У�����________�ⶨ��ӦҺ�����ȡ�
�ڴ�������ʹ������������—���������������������ͼ������ͨ��SO2��ˮ��Ŀ���ǡ�_____________________________________________________.���û�ѧ����ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��WΪ���ֶ���������Ԫ�أ�����X��Zͬ�壬Y��Zͬ���ڣ�W�Ƕ���������Ԫ����ԭ�Ӱ뾶���ģ�Xԭ�������������Ǻ�����Ӳ�����3����Y���������������۴�����Ϊ6������˵����ȷ����
A��YԪ������������Ӧ��ˮ���ﻯѧʽH2YO4
B��ԭ�Ӱ뾶�ɴ�С��˳��Ϊ��Z>Y> W
C��X��Z����Ԫ�ص���̬�⻯���У�Z����̬�⻯����ȶ�
D��X��W�γɵ����ֻ������У��������������ʵ���֮�Ⱦ�Ϊ1︰2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��50ml0.1mol/L��ˮ����ͨ������Cl2��ȫ��Ӧ��ת����0.05mol����,�����������������
A���÷�Ӧ��H2O�Ƿ�Ӧ��֮һ B���������뻹ԭ�������ʵ���֮��Ϊ1��5
C����������ΪHIO3 D������1 mol I2�μ�������Ӧ,ת��10 mol e-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA��ʾ�����ӵ���������ֵ������˵����ȷ���� �� ��
A����״���£�11.2 L SO3������ԭ����Ϊ2NA
B����״���£�22.4L��������Ȳ����������ķ�����ΪNA
C��23 g������������ȫȼ��ʧ������Ϊ0.5 NA
D��100 mL 2.0 mol/L��Na2S��Һ��S2-���Ӹ�������0.2 NA 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���л����ú��ȵ��л���A�ϳ���֯�����̿���������M����ƺϳ�·����ͼ(���ַ�Ӧ�Լ�������δע��)��

��֪�������������D����Է���������90��110֮�䣬����Ԫ�ص���������Ϊ0.615��
E��D����Է���������28���˴Ź���������ʾE��������2�ֲ�ͬ��������ԭ�ӣ���
������Ϊ3��1��
|

�ش��������⣺
(1)A�����к��������ŵ�������________��G��H�ķ�Ӧ������_______________________��
(2)D�ķ���ʽΪ_________��E�Ľṹ��ʽΪ__________________________________��
(3)H��J��Ӧ�Ļ�ѧ����ʽ��________________________________________��
(4)J��һ�������¿��Ժϳɸ߷��ӻ�����ø߷��ӻ�����Ľṹ��ʽ��______________��
(5)��֪1 mol E��2 mol J��Ӧ����1 mol M����M�Ľṹ��ʽ��___________________��
(6)д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽ��_______________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com