




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
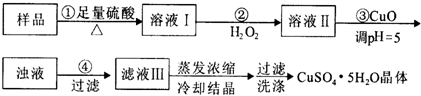
| A������CuCO3���CuOҲ�ɵ�����Һ��pH����Ӱ��ʵ���� |
| B��������з�������Ҫ��ӦΪ��H2O2+2Fe2++2H+=2Fe3++2H2O |
| C��ϴ�ӣ���װ�����©���м�ˮ����û���壬����Ȼ���º��ظ�2��3�� |
| D��ijʵ����Ҫ240 mL1mol/L��CuSO4��Һ��������ʱ�����CuSO4?5H2O 60g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
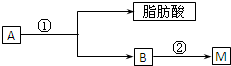
| Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
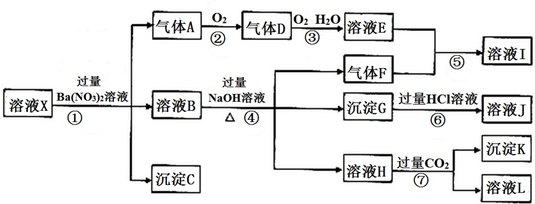
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�鲽�� | �й����� |
| �ټ�������NaCl������ | |
| �ڳ���NaCl���� | ��������ƽ��Ҫ����NaCl�������� |
| �۽�NaCl����100mL�ձ��У�����������ˮ | Ϊ�˼ӿ��ܽ����ʣ����Բ�ȡ��Щ��ʩ�� �ò��������Ͻ��裮 |
| �ܽ��ձ�����Һת����250mL����ƿ�� | Ϊ�˷�ֹ��Һ������Ӧ��ȡʲô��ʩ���ò����������� |
| ��������ƿ�м�����ˮ���̶��� | �ڽ��д˲���ʱ����ˮ���̶���1-2���״�Ӧ��β��������ý�ͷ�ιܼ�ˮ���̶��ߣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� ���� | ||||||||
| 1 | �� | |||||||
| 2 | �� | |||||||
| 3 | �� | �� | �� | �� | ||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ͬ������Ӧ�����ȶ��ı����� |
| B�������ȵ������� |
| C�����Ͻ�ǿ�ȴ� |
| D������ǿ��ԭ�������ȷ�Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ԫ��Z��W�ļ����ӵĵ��Ӳ�ṹ��ͬ |
| B��Ԫ�صķǽ�������ǿ������Y��Z��X |
| C��ֻ��X��Y��Z ����Ԫ�صĻ�������������ӻ����Ҳ�����ǹ��ۻ����� |
| D��Ԫ��Y����̬�⻯����������������Ӧ��ˮ���ﲻ��Ӧ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com