
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ʵ����� | Ԥ����������� |
| ����1�� | |
| ����2�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
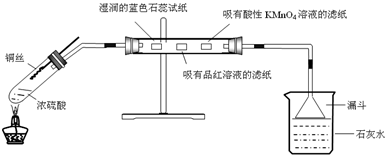
 2�����Ʊ���ijͬѧ�������һ���Ʊ�̼��Ƶķ�����������ͼΪ��
2�����Ʊ���ijͬѧ�������һ���Ʊ�̼��Ƶķ�����������ͼΪ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
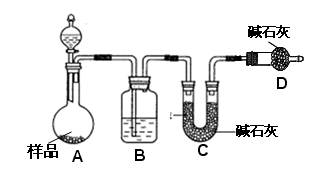
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
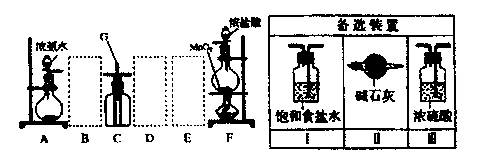
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
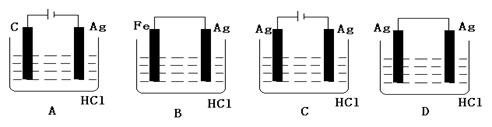
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������У�Ӧʹ������е�ˮδ��ȫ����ʱ����ֹͣ���� |
| B��������FeCl3��Һ������е�NaOH��Һ�У��Ӷ��Ƶ�Fe(OH)3���� |
| C�����˲��������������뽺�����Һ |
| D����ʼ����ʱ��Ӧ���ȼ���������ƿ���ٿ�����ˮ��������ϣ�Ӧ���ȹ�����ˮ�ٳ��ƾ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������������˻�������ַе�IJ�ͬ |
| B��ʳ��ˮ������������ˮ���������õ�������������̨���ƾ��ơ�ʯ������ |
| C����Һʱ������Һ©���ϿڵIJ�������Ŀ����Ϊ��ƽ���Һ©���������ѹ������Һ������ |
| D������ʱ����������������ֽһ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com