
| ʵ����� | Ԥ����������� |
| ����1�� | |
| ����2�� | |
| ʵ����� | Ԥ����������� |
| ����1��ȡ��������Һ�����Թܣ�����������ᣬ�����Ȼ�����Һ����2�֣� | �а�ɫ�������ɣ�֤������Һ�к�SO42-����1�֣� |
| ����2��ȡ��������Һ�����Թܣ����������ˮ����2�֣� | ��Һ�ʳȻ�ɫ��֤������Һ�к�Br-����1�֣� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������еμ���������Һ����������������к�����ԭ�� |
| B������֧�ֱ�ʢ�������������Ȼ�̼���Թ��мӵ�ˮ�������ֱ������Ȼ�̼ |
| C���ƾ����ˮ��ϲ�����ȡ��ˮ�еĵ� |
| D���ڱ��м����ۺ��ټ���ˮ������ȡ�屽 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
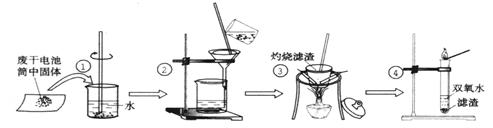
 H)2��������ϡ��ˮ���ɿ����Ե�Zn(NH3)4(OH)2�������Ǹ�ͬѧ�Բ����ڵ���Һ���γɷֽ���̽���Ĺ��̣�
H)2��������ϡ��ˮ���ɿ����Ե�Zn(NH3)4(OH)2�������Ǹ�ͬѧ�Բ����ڵ���Һ���γɷֽ���̽���Ĺ��̣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��Ҫ�ɷ�������������������������ˮ������
��Ҫ�ɷ�������������������������ˮ������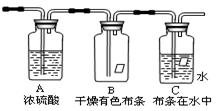
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 mol/L���ᡢ2mol/LNaOH��Һ��20%KSCN��Һ���������ʵ��̽�����̣�
mol/L���ᡢ2mol/LNaOH��Һ��20%KSCN��Һ���������ʵ��̽�����̣� ����Ԥ������ͽ��ۡ�
����Ԥ������ͽ��ۡ�| ��� | ʵ����� | ʵ������ | ���� |
| �� | ��ҩ��ȡ������Ʒ�������Թ�A�У����õι�ȡ����  ____ ��Һ���μӵ��Թ�A�У���ַ�Ӧ ____ ��Һ���μӵ��Թ�A�У���ַ�Ӧ | �й���ʣ�࣬�������ݲ��� | �Ͻ��г��������Fe��Cu Ԫ�� |
| �� | ���Թ�A��ʣ������мӹ��� ________ ����ַ�Ӧ���ã�ȡ�ϲ���Һ���Թ�B�� | ���岿���ܽ⣬��������ų�����Һ��dz��ɫ | |
| �� | ���Թ�B�м������� _____���ٵμ�KSCN��Һ | _____ | |
| �� | ����ʣ������м���ϡ����ٵμ� ____________ ��Һ. | �����ܽ⣬����ɫ�̼�������������ܿ��ɺ���ɫ����Һ����ɫ���ټ�ij��Һ������ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 CO2����2H2O��2SO2��
CO2����2H2O��2SO2��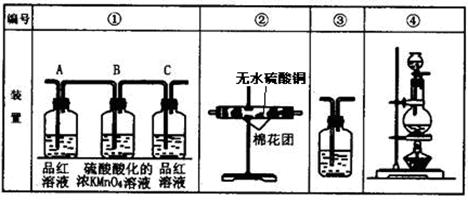
 ��˳���������������ҵķ����ǣ���װ�õı�ţ���
��˳���������������ҵķ����ǣ���װ�õı�ţ��� �����ܡ���__________��__________��__________��
�����ܡ���__________��__________��__________��| A��BaCl2��Һ | B��Ba(OH)2��Һ |
| C���μ�H2O2��BaCl2��Һ | D���μ�H2O2��Ba(OH)2��Һ |
 ��������Ϊbg����a��b��ʾCO2��SO2�����ʵ���������������������
��������Ϊbg����a��b��ʾCO2��SO2�����ʵ����������������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������C2H5Cl�е���Ԫ��ʱ����C2H5Cl��NaOH��Һ��ϼ��Ⱥ���ϡ���������ữ���ټ���������Һ |
| B������Һ�м������ᣬ����ɫ�����ݳ�����������ʹ����ʯ��ˮ����ǣ������Һ�к���CO32- |
| C����0.1mol��L��1 FeSO4��Һ�еμ���������KMnO4��Һ��KMnO4��Һ��ɫ��˵��Fe2+���������� |
| D����2.0mLŨ�Ⱦ�Ϊ0.1mol��L��1��KCl��KI�����Һ�еμ�1~2��0.01mol��L��1 AgNO3��Һ���������ʻ�ɫ��˵��AgCl ��Ksp��AgI ��Ksp�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ��Ӧ�� | I | II | III |
| �������x/ mL | 0<x��10.0 | 10.0<x��40.0 | x>40.0 |
| �� �� | ������ | ������ | ������ |
 �dz����� ����ʵ������Ҫ�ⶨ�������� ��
�dz����� ����ʵ������Ҫ�ⶨ�������� ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com