
����Ŀ���۴�����ϩ��E�Ǵ�����ϩ��D�ľۺ��Ӣ����дΪPVAc����Ҫ����Ϳ�ϣ�Ҳ��������ȡ����ϩ���;���ϩ����ȩ��ԭ�ϡ���ҵ�Ͽ������ºϳ�·����ȡ��
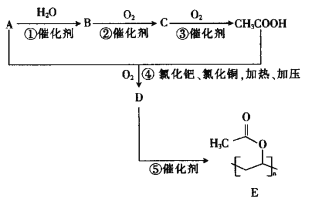
��֪��A�������ܶ�����������ܶ���14����ش��������⣺
��1��A�Ľṹ��ʽΪ__________��D�ķ���ʽΪ__________��B�й����ŵ�����Ϊ__________����Ӧ�ݵķ�Ӧ����Ϊ__________��
��2����ȫȼ�յ����ʵ�����A��B��C���������Ӵ�С��˳��Ϊ__________������ĸ��ʾ����
��3����Ӧ�ܵĻ�ѧ����ʽΪ________________________________________��
��4��D�ж���ͬ���칹�壬���ܷ���ˮ�ⷴӦ�����ܷ���������Ӧ��D��ͬ���칹����__________�֣����к˴Ź���������3��壬�ҷ����֮��Ϊ1��1��4��ͬ���칹��Ľṹ��ʽΪ__________��
��5����������֪ʶ����������Ϣ��д����CH3CH=CH2��CH3CH2CH2OHΪԭ���Ʊ�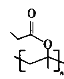 �ĺϳ�·������ͼ�����Լ����ã���____________________��
�ĺϳ�·������ͼ�����Լ����ã���____________________��
�ϳ�·������ͼʾ�����£�CH3CH2Br![]() CH3CH2OH
CH3CH2OH CH3COOCH2CH3
CH3COOCH2CH3
���𰸡�CH2=CH2 C4H6O2 �ǻ� �Ӿ۷�Ӧ A=B>C CH2=CH2+CH3COOH+O2 CH2=CH-OOCCH3+H2O 4
CH2=CH-OOCCH3+H2O 4 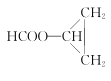 CH3CH2CH2OH
CH3CH2CH2OH![]() CH3CH2CHO
CH3CH2CHO![]() CH3CH2COOH
CH3CH2COOH![]() CH3CH2COOC��CH3��=CH2
CH3CH2COOC��CH3��=CH2![]()
��������
��֪A�������ܶ�����������ܶ���14����A��Ħ������Ϊ28g/mol��A��B��C��CH3COOH�����У�Cԭ����Ŀδ�䣬��A�ķ���ʽΪC2H4������ϩ����ȷ��BΪ�Ҵ���CΪ��ȩ��A�����ᷢ��ȡ����Ӧ����D��ΪCH2=CH-OOCCH3��D�����Ӿ۷�Ӧ����E��
��1��AΪ��ϩ����ṹ��ʽΪCH2=CH2��DΪCH2=CH-OOCCH3������ʽΪC4H6O2��BΪ�Ҵ������еĹ�����Ϊ�ǻ�����Ӧ��ΪD�ļӾ۷�Ӧ��
��2��A��B��C�ֱ�Ϊ��ϩ���Ҵ�����ȩ������ʽΪC2H4��C2H6O��C2H4O����Ϊ1molʱ���������ֱ�Ϊ3mol��3mol��2.5mol�����ɴ�С��˳��ΪA=B>C��
��3����Ӧ��Ϊ��ϩ�����ᡢ������Ӧ����CH2=CH-OOCCH3��ˮ������ʽΪCH2=CH2+CH3COOH+O2 CH2=CH-OOCCH3+H2O��
CH2=CH-OOCCH3+H2O��
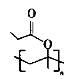
��4��D��ͬ���칹���У����ܷ���ˮ�ⷴӦ�����ܷ���������Ӧ���л����к�����������������һ�ˣ�ͬ���칹����CH2=CH-CH2OOCH��CH2=C��CH3��OOCH��CH3CH=CHOOCH��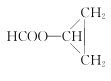 ������4�֣��˴Ź���������3��壬����2�����͵���ԭ�ӣ�������ֻ��6����ԭ�ӣ������֮��Ϊ
������4�֣��˴Ź���������3��壬����2�����͵���ԭ�ӣ�������ֻ��6����ԭ�ӣ������֮��Ϊ![]() �������Ϊ1��1��4���ṹ��ʽΪ
�������Ϊ1��1��4���ṹ��ʽΪ ��
��
��5���������̼���ѧ֪ʶ���Ʊ� ��������ȡ���ᣬ��ʹ�������ϩ����ȡ����Ӧ�����Ӿۣ����ɣ�����ΪCH3CH2CH2OH
��������ȡ���ᣬ��ʹ�������ϩ����ȡ����Ӧ�����Ӿۣ����ɣ�����ΪCH3CH2CH2OH![]() CH3CH2CHO
CH3CH2CHO![]() CH3CH2COOH
CH3CH2COOH![]() CH3CH2COOC��CH3��=CH2
CH3CH2COOC��CH3��=CH2![]()
 ��
��


 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ģ�[��ѧ����ѡ��5���л���ѧ����]
�����б��������������E�뻯����H��Cr-Ni���¿��Է���ż����Ӧ���ϳ�һ�ֶ�����ŵĻ�����Y����ϳ�·�����£�

��֪��![]()
�ش��������⣺
��1��A�Ļ�ѧ������________________��
��2��BΪ���ȴ�������B����C�Ļ�ѧ����ʽΪ________________��
��3����A����B��G����H�ķ�Ӧ���ͷֱ���________________��________________��
��4��D�Ľṹ��ʽΪ________________��
��5��Y�к��������ŵ�����Ϊ________________��
��6��E��F��Cr-Ni����Ҳ���Է���ż����Ӧ������Ľṹ��ʽΪ________________��
��7��X��D��Ϊͬ���칹�壬�Ҿ�����ȫ��ͬ�����š�X�ĺ˴Ź���������ʾ���ֲ�ͬ��ѧ�������⣬������֮��Ϊ3��3��2��д��3�ַ�������������X�Ľṹ��ʽ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ���� �� ��
A. ����AgCl��AgI���������Һ��c(Ag��)��c(C1��)��c(I��)
B. �ں���BaSO4��������Һ�м���Na2SO4���壬c(Ba2��)����
C. AgCl��ͬŨ�ȵ�CaCl2��NaCl��Һ�е��ܽ����ͬ
D. CaCO3 ������ϡ���ᣬ�������ڴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ����գ�
I.��1��AlCl3��ˮ��ҺpH______7�����=����������ԭ��Ϊ______________�������ӷ���ʽ��ʾ����������Һ�������ɲ��������յõ�������_____________________���ѧʽ����
��2�������£�Cr(OH)3���ܶȻ�Ksp=c(Cr3+)��c3(OH)=1032��Ҫʹc(Cr3+)����105mol/L����Һ��pHӦ����___��
��3�����ʵ���Ũ����ͬ��������Һ����(NH4)2SO4 �ڰ�ˮ ��NH4HSO4��c(NH4+)��С˳����ȷ����______��������ű�ʾ��
��4��������������Һ��a��pH=4 NH4Cl b��pH=4������Һ������ˮ�����C��H+��֮��Ϊ______��
II�������£�ijһԪ����HA�ĵ��볣��K��1.6��10��6�� ��20.00 mL Ũ��ԼΪ0.1 mol��L��1 HA��Һ����μ���0.1000 mol��L��1 �ı�NaOH��Һ����pH�仯������ͼ��ʾ(�����¶ȱ仯)����ش������й����⣺
��1��a��b��c��d�ĵ���ˮ�ĵ���̶�������________�㣬�ζ���������ѡ��________��ָʾ�����ζ��յ���___(�c�����ϡ���c�����¡�)��
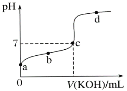
��2���ζ������в��ֲ������£����и�����ʹ�������ƫ�ߵ���___________������ĸ��ţ���
A���ζ�ǰ��ʽ�ζ���δ�ñ�NaOH��Һ��ϴ
B��������ˮϴ����ƿ������װ��HA��Һ����еζ�
C���ζ������У���Һ���ֱ�ɫ������ֹͣ�ζ�
D���ζ�����������Һ�棬��ȡNaOH��Һ���
��3�����ظ����εζ�ʵ����������±���ʾ������ζ�����HA��Һ�����ʵ���Ũ��Ϊ_______mol/L��������4λ��Ч���֣�
ʵ����� | NaOH��Һ���/mL | ����HA��Һ���/mL |
1 | 21.01 | 20.00 |
2 | 20.99 | 20.00 |
3 | 21.60 | 20.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Na2CO3��NaHCO3���Һ����μ���ϡ���ᣬ�������������ϡ����������ı仯��ϵ����ͼ��ʾ���������������ڶ�Ӧ����Һ��һ���ܴ���������ǣ� ��
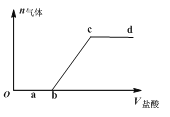
A.a���Ӧ��Һ�У�Ca2����Mg2����Br����NO3��
B.b���Ӧ��Һ�У�Al3����Fe3����MnO4����NO3��
C.c���Ӧ��Һ�У�Na����Ca2����NO3����Cl��
D.d���Ӧ��Һ�У�F����NO3����Fe2����Ag��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ����������(��Ҫ�ɷ�ΪAl2O3����Fe2O3����)Ϊԭ��ұ�����Ĺ����������£�

����������ȷ����
A. �Լ�X����������������Һ��Ҳ����������
B. ��Ӧ�������˺����ó���Ϊ��������
C. ͼ����ʾת����Ӧ������������ԭ��Ӧ
D. ��Ӧ���Ļ�ѧ����ʽΪNaAlO2��CO2��2H2O=Al(OH)3����NaHCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ֿ��������쵼�����״��ֺ��Աʹ�õ�����ƿ���Ʊ�����һ���䷽�к��������������ʣ�

��д���пհף�
��1�����в�����ԭ�ӵĹ�������___�������Լ������Ӧ����ɫ����___(����ĸ)��
a��Br2/CCl4��Һ b��ʯ����Һ c������KMnO4��Һ
��2����ͬ���칹���ж��֣�д������һ�ֲ�����������Ľṹ��ʽ��____��
��3����֪��C2H5Br��NaOH![]() CH3CH2OH��NaBr������ͨ������ת�����Եõ���(CH2OHCH2OH)��
CH3CH2OH��NaBr������ͨ������ת�����Եõ���(CH2OHCH2OH)��
����![]() A
A![]() B(C2H5OH)
B(C2H5OH)![]() C(C2H4)
C(C2H4)![]() D(C2H4Br2)
D(C2H4Br2)![]() ��
��
��A�ķ���ʽ��____���Լ�X������_____��
��д����D�����һ�ѧ����ʽ��_______��
��4����֪��
 +HCl��-RΪ�ǻ���
+HCl��-RΪ�ǻ���
 +H2
+H2
��������֪ʶ����������Ϣ��д���Ա�����ϩ���Ȼ���Ϊԭ���Ʊ����ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
CH3CH2Br![]() CH3CH2OH
CH3CH2OH![]() CH3COOCH2CH3______��
CH3COOCH2CH3______��
��5��д�����������������������ɸ߷��ӻ�����Ļ�ѧ����ʽ��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ�������жϴ�����ǣ� ��
A.NA��H��������Ϊ1 g
B.46g NO2��N2O4�������������ԭ����ĿΪ2NA
C.�ڳ��³�ѹ�£�11.2L N2���еķ�����С��0.5NA
D.56g�������������ᷴӦ��ת�Ƶĵ���Ϊ3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�Ǻϳɰ���Ӧ�������淴Ӧ�����淴Ӧʱ��仯��ʾͼ���й������������
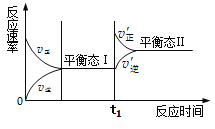
A. ״̬���״̬��ʱ����Ӧ������ƽ��״̬
B. ״̬��仯Ϊ״̬��Ĺ��̣���Ϊ��ѧƽ���ƶ�
C. t1ʱ��ƽ��������Ӧ�����ƶ���ƽ�ⳣ������
D. ͬһ�ַ�Ӧ����״̬I��״̬IIʱ��Ũ�Ȳ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com