
����Ŀ������ѧ����ѡ��2��ѧ�뼼������������ͭ�̳������������ս�ȡ���ȿɱ��������ֿɵõ���;�㷺�ĸߴ�����п����֪�̳�����Ҫ��ZnO������������FeO��Fe2O3��CuO��MnO��PbO��CdO���Ʊ������������£�
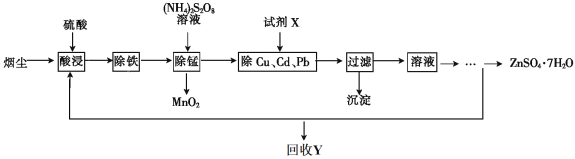
��֪����ؽ������������������������pH��������ȫ��pH���±���
�������� | ��ʼ������pH | ������ȫ��pH |
Fe3+ | 1��1 | 3��2 |
Zn2+ | 5��5 | 8��0 |
Fe2+ | 5��8 | 8��8 |
��1�����յIJ�ƷY��_____________________��
��2����������ʱ���ȼ���_________________������������ԭ�������ٽ���Һ��pH������__________��
��3�������̡�ʱ��Һ�з�����Ӧ�Ļ�ѧ����ʽΪ____________________��
��4����ȥCu��Cd��Pb��ͬʱ���Ի���һЩ�ؽ������Լ�XΪ__________________��
��5��ZnSO4��Һ����__________��__________�����˼�����õ�ZnSO4��7H2O��
��6��Ϊ�˲ⶨ��Ʒ��ZnSO4��7H2O�ĺ�����ȡag��Ʒ����ˮ����������Һ����������̼������Һ��������ȫ�����ˡ�ϴ�ӳ������ڽ�����������������ȫ�ֽ⣬�Ƶ�Ϊbg�����Ʒ��ZnSO4��7H2O������������__________________________��
���𰸡�
��1����NH4��2SO4 ��2�֣�
��2����������1�֣� 3��2~5��5��1�֣�
��3��MnSO4+��NH4��2S2O8+2H2O![]() MnO2��+��NH4��2SO4+2H2SO4��3�֣�
MnO2��+��NH4��2SO4+2H2SO4��3�֣�
��4��Zn�ۣ�1�֣�
��5������Ũ����2�֣� ��ȴ�ᾧ��2�֣�
��6����100%��3�֣�
��������
���������
��1�����ڡ����̡����������ӵ�����NH4��2S2O8��������õ������ᣬ��Y�ǣ�NH4��2SO4��
��2����������ʱ��Ӧ�ȼ�����������Fe2+����ΪFe3+��������Һ��pHӦ������3��2~5��5��ʹ��ȫ������������
��3����MnO������ʱ����MnSO4��������ʱ����Ӧ����ʽ�ǣ�
MnSO4+��NH4��2S2O8+2H2O =MnO2��+��NH4��2 SO4+2H2SO4��
��4����ȥCu��Cd��Pb��ͬʱ���Ի���һЩ�ؽ���������Cu2+��Cd2+��Pb2+ת��ΪCu��Cd��Pb���ʣ��ʼ���Zn�ۡ�
��5������Ũ������ȴ�ᾧ
��6���������غ㣺ZnSO4��7H2O~ZnO���г�287��81=x��b��x=������Ʒ��ZnSO4��7H2O��������������100%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�С�����̼�����Ƶ��Ʊ�ʵ�顣�йط�Ӧ�Ļ�ѧ����ʽΪ��
NH3��CO2��H2O=NH4HCO3 ��NH4HCO3��NaCl=NaHCO3����NH4Cl
��1��һλͬѧ��������̼����ͨ�뺬���ı���ʳ��ˮ���Ʊ�̼�����ƣ�ʵ��װ������ͼ��ʾ(ͼ�мг֡��̶��õ�����δ����)���Իش������й����⣺
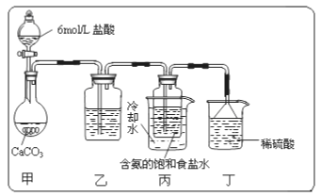
��ʵ�����ư����Ļ�ѧ����ʽ__________________��
����װ���е��Լ���______����װ����ϡ�����������____________��
��ʵ����������NaHCO3����IJ�����____________(��������������)���ò�������Ҫ�IJ��������У���������____________��
��2��̼�������������ù���12.28g ��������ʯ��ˮ��ַ�Ӧ�����ó�����ϴ�ӡ��������Ϊ 12.00g,�����ù�����̼���Ƶ���������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼���Ƶ���;�ܹ㣬������ұ�𡢷�֯��ƯȾ�ȹ�ҵ�Ļ���ԭ�ϡ����������ش��������⣺
��.�������繤ҵ����̼���Ƶķ�����·����(N.Leblanc)�������������£�
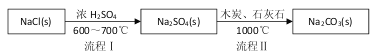
��1������I����һ������____���������ķ�Ӧ�ֲ����У�a. ![]()
b.Na2S��ʯ��ʯ�������ֽⷴӦ���ܷ�Ӧ����ʽ�ɱ�ʾΪ__________________��
��1862�꣬����ʱ������ά(ErnestSolvay)�ð������̼���ơ���Ӧԭ�����£�
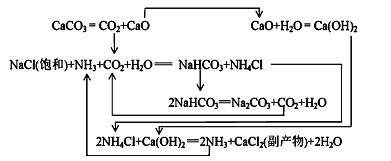
20��ʱһЩ������ˮ�е��ܽ��/g(100gH2O)
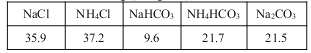
��2��������ɴ����ԭ����____________����ѭ�����õ�������____________��
��3������NaCl��ҺͨNH3��CO2������NaHCO3��ԭ���У�_________��__________��_________��
���ҹ�����ר�Һ�°��о��������Ƽ���䷴Ӧԭ���Ͱ�����ƣ������ư����Ƽ����ϣ������ԭ�������ʡ�
��4����������������NaHCO3�����õ���Һ�м���NaCl���岢ͨ��NH3����________(���¶ȷ�Χ)������________(�ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ⱻ��Ϊ��ɫ�����������ķ��ӽṹ����ͼ��ʾ����֪�÷���H��O��O���Ƕ���97�㡣�����й�H2O2��˵����ȷ����
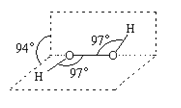
A.������������д������Ӽ������ۼ�
B.��1.00mol H2O2�У������������Ϊ10��6.02��1023��
C.��������������������л�ԭ��
D.3.4g���������к���6.02��1022��ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������[Al��NO3��3]��һ�ֳ���ýȾ������ҵ�������ң���Ҫ��Al��Al2O3��Fe2O3�ȣ���ȡ����������[Al��NO3��39H2O]��������ͼ�ף�
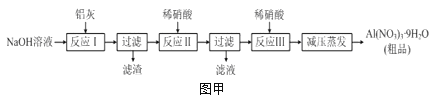
��1��д����Ӧ�������ӷ���ʽ��______________________________________________��
��2������ͼ����ʾʵ��װ����ȡAl��NO3��3��ͨ��ˮ������������__________________��
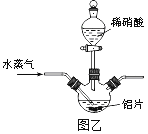
��3�����������в��ü�ѹ�����������Ʒ�Ӧ���м����ϡ�����Թ�������Ŀ����__________��
��4���¶ȸ���200��ʱ����������ȫ�ֽ����������ij���塣��֪��2NO2+2NaOH=NaNO2+NaNO3��Ϊ��ȷ����������ijɷ֣�ijѧ����������װ�ý���ʵ�顣
������ʵ��ʱװ���ӿ���ȷ˳����A��________________________��F��
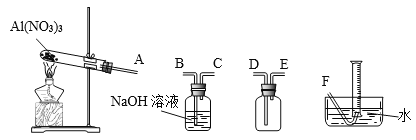
��֤���ֽ����ɵĻ����������NO2��������_____________________��
��ȡ21��3g Al��NO3��3���ȷֽ⣬����ˮ���ռ����壬�����ڱ�״�����ռ���_____mL���壬�����ѧ����ʽ˵������____________________________��
��5�����ʵ��֤����Ʒ�к���Al��NO3��3��_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��̽����ѧ��Ӧ���ʺͻ�ѧ��Ӧ�ȵ��й����⣬ij�о�С�����������ʵ�飺
��.��H2O2�ķֽⷴӦΪ�о�����ʵ�鷽�������ݼ�¼���±���t��ʾ�ռ�a mL O2�����ʱ�䡣
��� | ��Ӧ �¶�/�� | c(H2O2)/ mol��L��1 | V(H2O2) /mL | m(MnO2) /g | t/min |
1 | 20 | 2 | 10 | 0 | t1 |
2 | 20 | 2 | 10 | 0.1 | t2 |
3 | 20 | 4 | 10 | 0.1 | t3 |
4 | 40 | 2 | 10 | 0.1 | t4 |
��1�����ʵ��2��ʵ��3��Ŀ�����о�____________�Ի�ѧ��Ӧ���ʵ�Ӱ�졣
��2��Ϊ�о��¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬���Խ�ʵ��________��ʵ��________���Ա�(�����)��
��3����ʵ��1��ʵ��2���Աȣ�t1________t2(������������������������)��
��.��KI��FeCl3��ӦΪ��(2Fe3����2I��![]() 2Fe2����I2)���ʵ�飬̽���˷�Ӧ����һ�����ȡ���ѡ�Լ���
2Fe2����I2)���ʵ�飬̽���˷�Ӧ����һ�����ȡ���ѡ�Լ���
��0.1 mol��L��1 KI��Һ ��0.1 mol��L��1 FeCl3��Һ ��0.1 mol��L��1 FeCl2��Һ ��0.1 mol��L��1 ���� ��0.1 mol��L��1 KSCN��Һ ��CCl4
ʵ�鲽�裺��1��ȡ5 mL 0.1 mol��L��1 KI��Һ���ٵμӼ���0.1 mol��L��1 FeCl3��Һ��
��2����ַ�Ӧ����Һ�ֳ�������
��3��ȡ����һ�ݣ����Լ�������CCl4������ɫ��˵����Ӧ���ɵ���
��4����ȡһ�ݣ����Լ�________(�����)������______________��˵���˷�Ӧ����һ�����ȡ�
��.N2O4�ɷֽ�ΪNO2����100 mL�ܱ�������Ͷ��0.01 mol N2O4�������ִ���ѧʵ�鼼�����ٲ���c(NO2)��c(NO2)��ʱ��仯�����ݼ�¼����ͼ��ʾ��
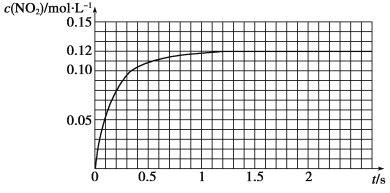
��1����Ӧ���������ʣ���������______________������N2O4�����ʵ���Ϊ________mol��
��2��c(NO2)��ʱ��仯�����߱�����ʵ���õĻ�ѧ��Ӧ��������С�����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��8���л��������ת����ϵ��
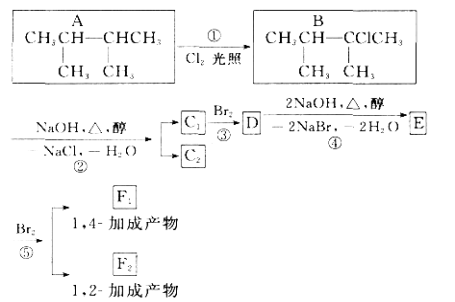
��ش��������⣺
��1��������ͼ�У�����________��Ӧ������________��Ӧ(�Ӧ����)��
��2��������E����Ҫ�Ĺ�ҵԭ�ϣ�д����D����E�Ļ�ѧ����ʽ��_______________________________________________________________��
��3�� C 1�Ľṹ��ʽ��______________________��F 1�Ľṹ��ʽ��_____________________________��
��4�� ����8�ֻ������У����ڶ�ϩ������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʩ������Schlesinger�������������NaBH4��ˮ��Ӧ��ȡ������BH4�� + 2H2O == BO2�� + 4H2������Ӧʵ��Ϊˮ���������H+����ԭ�����о��������÷�Ӧ����H2���������������Ӱ�죬�±�ΪpH���¶ȶ�NaBH4��˥�ڵ�Ӱ�죨��˥����ָ��Ӧ�����У�ij���ʵ�Ũ�Ƚ��͵���ʼŨ��һ��ʱ�����ʱ�䣩��
��ϵ pH | ��ͬ�¶��µİ�˥�ڣ�min�� | |||
0�� | 25�� | 50�� | 75�� | |
8 | 4.32��100 | 6.19��10��1 | 8.64��10��2 | 1.22��10��2 |
10 | 4.32��102 | 6.19��101 | 8.64��100 | 1.22��100 |
12 | 4.32��104 | 6.19��103 | 8.64��102 | 1.22��102 |
14 | 4.32��106 | 6.19��105 | 8.64��104 | 1.22��104 |
��1����֪NaBH4��ˮ��Ӧ��������Һ�Լ��ԣ������ӷ���ʽ��ʾ����Һ�Լ��Ե�ԭ�� ����Һ�и�����Ũ�ȴ�С��ϵΪ ��
��2�����ϱ���֪���¶ȶ�NaBH4��ˮ��Ӧ���ʲ���������Ӱ�죿 ��
��3����Ӧ��ϵ��pHΪ�λ��NaBH4��ˮ��Ӧ�ķ�Ӧ���ʲ���Ӱ�죿 ��
�����£�N2H4���ֳ���������������һ����ɫ��״Һ�壬�е�Ϊ113.5�����º������ڲ�ͬ�¶Ⱥʹ������������ɲ�ͬ�����ͼ����

�¶Ƚϵ�ʱ��Ҫ��Ӧ����N2H4 + O2 ![]() N2 + 2H2O
N2 + 2H2O
�¶Ƚϸ�ʱ��Ҫ��Ӧ����N2H4 + 2O2 ![]() 2NO + 2H2O
2NO + 2H2O
������������Ӧ�����������գ�
��4������Ӧ����250��ʱ��ƽ�ⳣ��ΪK1��350��ʱ��ƽ������ΪK2����K1 K2�����������������������
��5����Ӧ��1100��ʱ�ﵽƽ������д�ʩ��ʹ������![]() ������� ������ĸ��ţ���
������� ������ĸ��ţ���
A�����������£�����He��
B�������������
C�����������£�����N2H4
D��ʹ�ô���
��6������n mol�º�2n molO2����ij�ݻ�Ϊn L�ĸ��������У���800����һ��ѹǿ�����ʴ����������£���Ӧ������ͬʱ�ﵽƽ�⣬ʵ����N2�IJ���x��NO�IJ���Ϊy����������·�Ӧ����ƽ�ⳣ��K= ����x��y�Ĵ���ʽ��ʾ�����ػ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������, ��ѧ��������һ�����͵��Ҵ���أ�DEFC��, ���û������������ܼ�, ��200������ʱ����, �Ҵ���رȼ״����Ч�ʸ߳�32���Ҹ��Ӱ�ȫ������ܷ�ӦʽΪ: C2H5OH+3O2 ![]() 2CO2+3H2O������˵������ȷ����
2CO2+3H2O������˵������ȷ����
A��C2H5OH�ڵ�صĸ����ϲμӷ�Ӧ B��1 mol�Ҵ�������ת��6 mol����
C�������·�е����ɸ����ص����������� D����������õ��ӵ�������O2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com