
���� ��1��ѡ��500 mL����ƿ�����ƴ���Խ�࣬��������Խ��
��2������c=$\frac{1000�Ѧ�}{M}$����Ũ�������ʵ���Ũ�ȣ��ٸ���ϡ�Ͷ��ɼ�����ҪŨ�������������ȷ����Ͳ���
��3����Ͳ�����ʹ��ȡ��Ũ���ᱻϡ�ͣ������Ҫ��ˮ���ݣ�����ƿ��������Ӱ�죻
��4����Һ��ú�ʱ�䲻�ã�Ӧ�����ϸ��ƿ�ڲ����ϱ�ǩ��
��5��û����ȴ��ת�Ƶ�����ƿ�У��ᵼ����ҺŨ��ƫ��
��� �⣺��1�����ƴ���Խ�࣬��������Խ��ѡ��200 mL��250 mL������ƿ��һ������ȷ��Ӧѡ��1��500 mL����ƿ��
�ʴ�Ϊ������ȷ��1��500 mL����ƿ�����ƴ���Խ�࣬��������Խ��
��2��Ũ�������ʵ���Ũ��Ϊ$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L������ϡ�Ͷ��ɣ���ҪŨ�������Ϊ$\frac{500mL��1.0mol/L}{18.4mol/L}$=27.2mL����Ҫѡ��50 mL����Ͳ��
�ʴ�Ϊ��50 mL��27.2��
��3����Ͳ�����ʹ��ȡ��Ũ���ᱻϡ�ͣ�������ҺŨ��ƫС�������Ҫ��ˮ���ݣ�����ƿ��������Ӱ�죬��ͲΪ����ʽ����������Ҫϴ�ӣ�
�ʴ�Ϊ����Ͳ������ʹ���ƫС��������ƿ��������Ӱ�죻����
��4������ƿ���ܳ�ʱ��ʢ����Һ���ᵼ��Ӱ�쾫�ȣ����ƺõ���Һ�������Լ�ƿ�У������ϱ�ǩ��ע���������ڡ�Ũ�ȼ������������
�ʴ�Ϊ�����ƺõ���Һ�������Լ�ƿ�У������ϱ�ǩ��ע���������ڡ�Ũ�ȼ������������
��5���������������е���©֮����û����ȴ��ת�Ƶ�����ƿ�У���ȴ����Һ���ƫС���ô���©�����������ҺŨ��ƫ��
�ʴ�Ϊ��û����ȴ��ת�Ƶ�����ƿ�У�ƫ��
���� ���⿼����Һ�����ƣ�ע�����c=$\frac{n}{V}$������Һ����ԭ������������ע��Ũ�����������ʵ���Ͳ����Һ��ú�ʱ�䲻�ã�Ӧ�����ϸ��ƿ�ڲ����ϱ�ǩ��


 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
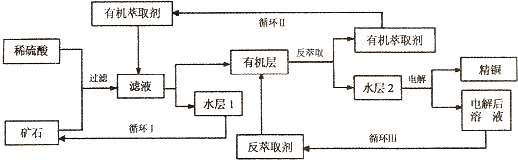
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | a=1��b=2 | B�� | a=2��b=1 | C�� | a=2��b=2 | D�� | a=3��b=2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶��С˳��D��C��B��A | |
| B�� | ��B��CԪ����ɵĻ�������ԼȺ������Ӽ����ֺ��й��ۼ� | |
| C�� | Ԫ��B��D��E�ֱ���A�γɵĻ������У��۷е���͵���B��A�γɵĻ����� | |
| D�� | Ԫ��D��C�γɵĻ������ڿ����г��ڷ��ò��ױ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | S��Cu��Ӧ��Cu+S$\frac{\underline{\;\;��\;\;}}{\;}$CuS | |
| B�� | ������ù�����pH��С��2H2SO3+O2�T2H2SO4 | |
| C�� | �����������Һ������ʯ��ˮ��ϣ�Ca2++HSO3-+OH-�TCaSO3��+H2O | |
| D�� | ��Na2S��Na2SO3�Ļ����Һ�еμ�ϡH2SO4[n��Na2S����n��Na2SO3��=2��1]��2S2-+SO32-+6H+�T3S��+3H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | m+n��p | B�� | C���������������� | ||
| C�� | ƽ��������Ӧ�����ƶ� | D�� | B��ת���ʼ�С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
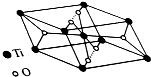 ��1������˵����ȷ����AD������ţ�
��1������˵����ȷ����AD������ţ� ��Ni����CO�γ����������ε������Ni��CO��4��1 mol Ni��CO��4�ĺ��Ц� ������ĿΪ8NA��
��Ni����CO�γ����������ε������Ni��CO��4��1 mol Ni��CO��4�ĺ��Ц� ������ĿΪ8NA���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
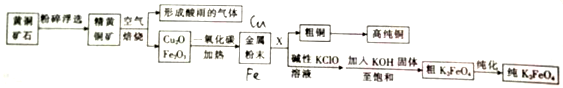
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��pH=1����Һ�У�K+��Na+��SO42-��HCO3- | |
| B�� | ��0.1 mol•L-1 Na2CO3��Һ�У�Al3+��K+��NO3-��SO42- | |
| C�� | ��0.1 mol•L-1 FeCl3��Һ�У�K+��NH4+��I-��SCN- | |
| D�� | ��$\frac{c��{H}^{+}��}{c��OH��}$=10-12����Һ�У�K+��Na+��ClO-��NO3- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com