
 HCOOCH3(g)+2H2(g) ��H>0
HCOOCH3(g)+2H2(g) ��H>0 CH3OH(g) +CO(g) ��H>0
CH3OH(g) +CO(g) ��H>0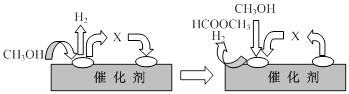


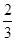 +�ڡ�
+�ڡ�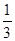 ���ܵø÷���ʽ�����ݸ�˹���ɿɸ÷�Ӧ�ȡ�H��(2a+b)/3 kJ��mol��1��
���ܵø÷���ʽ�����ݸ�˹���ɿɸ÷�Ӧ�ȡ�H��(2a+b)/3 kJ��mol��1��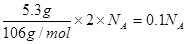 ��
��

 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���кͷ�Ӧ�����ȷ�Ӧ |
| B��ȼ���Ƿ��ȷ�Ӧ |
| C����ѧ�����ѷų����� |
| D����Ӧ����������������������һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������91��5kJ | B������183kJ | C������183kJ | D������91��5kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ���� | H2(g) | Br2(g) | HBr(g) |
| 1 mol�����л�ѧ������ʱ��Ҫ���յ�����/kJ | 436 | 200 | 369 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ˮ���⣺2H2O���2H2����O2�� |
| B������ʹˮ�ֽ����⣺2H2O����2H2����O2�� |
C��̫������ֽ�ˮ���⣺2H2O 2H2����O2�� 2H2����O2�� |
| D����Ȼ�����⣺CH4��H2O����CO��3H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH(g)�����ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH(g)�����ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
 ��ԭΪN2��һ��ʱ�����Һ�ļ���
��ԭΪN2��һ��ʱ�����Һ�ļ��� ��ԭ����ͼ��ʾ����Դ����Ϊ ���a����b������
��ԭ����ͼ��ʾ����Դ����Ϊ ���a����b������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���


�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com