
| A�����Ӽ� | B����λ�� | C���Ҽ� | D���м�E����� |





| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ���ۼ� | C��C | C��N | C��S |
| ����/(kJ��mol-1) | 347 | 305 | 259 |
 ����CH3(��)��C
����CH3(��)��C ������Ҫ���л���Ӧ�м���,�й����ǵ�˵������ȷ����(����)
������Ҫ���л���Ӧ�м���,�й����ǵ�˵������ȷ����(����) ��NH3��H3O+��Ϊ�ȵ�����,���ι��;�Ϊ������
��NH3��H3O+��Ϊ�ȵ�����,���ι��;�Ϊ������ �е�̼ԭ�Ӳ�ȡsp2�ӻ�,����ԭ�Ӿ�����
�е�̼ԭ�Ӳ�ȡsp2�ӻ�,����ԭ�Ӿ����� ��һ��C
��һ��C ��Ͼ��ɵõ�CH3CH3
��Ͼ��ɵõ�CH3CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
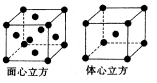
| A�����Ǿ��ɼ���ȥ��һ����ԭ������ |
| B�����ǻ�Ϊ�ȵ����壬̼ԭ�Ӿ���ȡsp2�ӻ� |
| C��CH3-��NH3��H2O+��Ϊ�ȵ����壬���ι��;�Ϊ������ |
| D��CH3+�е�̼ԭ�Ӳ�ȡsp2�ӻ�������ԭ�Ӿ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
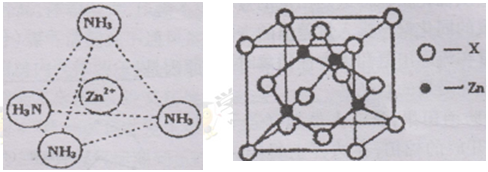
| Ԫ�� | �����Ϣ |
| A | Aԭ�ӵĺ�����������������̬���Ӳ��� |
| B | Bԭ�ӵ������������Ǵ�����������2�� |
| C | C�Ļ�̬ԭ��L���Ӳ�����3��δ�ɶԵ��� |
| D | D����Χ���Ӳ��Ų�Ϊ(n+1)d3n(n+2)sn |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A���Ȼ��(NH4Cl)�ĵ���ʽ�� |
B��H2O2�ĵ���ʽ�� |
| C��HClO�Ľṹʽ��H��O��Cl |
D��14C��ԭ�ӽṹʾ��ͼ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com