
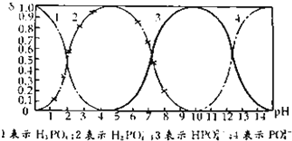
 ���ᣨH3PO4������Һ���ܹ���H3PO4��H2PO4-��HPO42-��PO43-�������ӵ���ʽ���ڣ�����Һ�е�pH�����仯ʱ��������һ�����ӵ����ʵ���ռ�������������ʵ����ķ�����Ҳ���ܷ����仯����ͼ��ʾ��H3PO4��Һ�У��������ӵ����ʵ�����������pH�仯���ߣ�
���ᣨH3PO4������Һ���ܹ���H3PO4��H2PO4-��HPO42-��PO43-�������ӵ���ʽ���ڣ�����Һ�е�pH�����仯ʱ��������һ�����ӵ����ʵ���ռ�������������ʵ����ķ�����Ҳ���ܷ����仯����ͼ��ʾ��H3PO4��Һ�У��������ӵ����ʵ�����������pH�仯���ߣ�| c(Na+) |
| c(PO43-) |
| c(Na+) |
| c(PO43-) |
| c(Na+) |
| c(PO43-) |


 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Mg��Al��ĩ���Ȼ�ϣ����뵽100mLijŨ�ȵ������У������������������״���£�������ĩ��������ϵ��ͼ��ʾ��
��Mg��Al��ĩ���Ȼ�ϣ����뵽100mLijŨ�ȵ������У������������������״���£�������ĩ��������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪ij��ȼ�Ϻ���̼���⡢������Ԫ�أ�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������������ȼ�գ���ʹ����������ȫ��ͨ����ͼ��ʾ��װ�ã��õ����±����е�ʵ���������������������ȫ�����գ���
��֪ij��ȼ�Ϻ���̼���⡢������Ԫ�أ�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������������ȼ�գ���ʹ����������ȫ��ͨ����ͼ��ʾ��װ�ã��õ����±����е�ʵ���������������������ȫ�����գ���| ʵ��ǰ | ʵ��� | |
| �������+U�ιܣ������� | 101.1g | 102.9g |
| ������ʯ��ˮ+���ƿ�������� | 312.0g | 314.2g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪һ�������л���A����������ȫȼ�գ�ֻ����0.2mol CO2��0.3mol H2O���Իش��������⣺
��֪һ�������л���A����������ȫȼ�գ�ֻ����0.2mol CO2��0.3mol H2O���Իش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������£�20L pH=12��Na2CO3��Һ�к��е�OH-������Ϊ0.2NA | ||
| B���к͵�����������ʵ���Ũ������ʹ�����Һ����������NaOH��Һ���ڴ��� | ||
C����0.1mol/L NH3?H2O��Һ�м�������NH4Cl�� �壬��Һ��
| ||
| D��һ���¶��£�10mL 0.50mol?L-1 NH4Cl��Һ��20mL 0.25mol?L-1 NH4Cl��Һ��NH4+���ʵ�����ͬ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com