
����Ŀ��ijǿ������ɫ��Һ�п��ܺ��±��е����������ӡ�
������ | Mg2+��NH4+��Ba2+��Al3+��Fe2+ |
������ | SiO32-��MnO4����Cl����NO3����SO32���� |
ʵ���ȡ��������Һ��������ʵ�顣
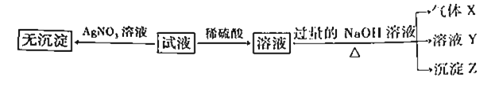
ʵ���Ϊ�˽�һ��ȷ������Һ����ɣ�ȡ100mLԭ��Һ�������Һ�еμ�1mol��L-1��NaOH��Һ����������������������������Һ����Ĺ�ϵ��ͼ��ʾ��

�ش��������⣺
��1��������ʵ��Ϳ����ƶϳ����ϱ��е�����һ�������ڵ���____________�֡�
��2��ͨ��ʵ������ȷ������Һ��һ�����ڵ���������________________��
��3��д��ʵ����ͼ����BC�ζ�Ӧ�����ӷ���ʽ��_______________________________________________________________��
��4��A���Ӧ�Ĺ�������Ϊ____________g��
��5������Һ�������ӵ�Ũ��Ϊ____________mol��L��1
���𰸡�4NO3��Al(OH)3��OH��=Al[(OH)4]����Al(OH)3��OH��=AlO2����2H2O0.1360.08
��������
ijǿ������ɫ��Һ��Fe2����SiO32-��MnO4-��SO32-һ�������ڣ���Һ����������������ְ�ɫ����������һ������Cl-��������������������һ������Ba2+������������������ƣ����ȣ��������������壬��һ������Mg2+��NH4+��Ϊ�˽�һ��ȷ������Һ����ɣ�ȡ100mLԭ��Һ�������Һ�еμ�1molL-1��NaOH��Һ�����ݲ�������������������������Һ����Ĺ�ϵ���õ�һ������Al3+�����ݴ��ڵ������Լ�������������ݵ���غ�ȷ�����������Ƿ���ڣ��ݴ˽��
��1��ijǿ������ɫ��Һ��Fe2����SiO32-��MnO4-��SO32-һ�������ڣ���һ�������ڵ���4�֣�
��2��ǿ������ɫ��Һ��Fe2����SiO32-��MnO4-��SO32-һ�������ڣ���Һ����������������ְ�ɫ����������һ������Cl-��������������������һ������Ba2+������������������ƣ����ȣ��������������壬��һ������Mg2+��NH4+��������Һ�Ե����Կ�֪����Һ��һ�����ڵ���������NO3-��
��3��ʵ����ͼ����BC�����������������������ƵĹ��̣���Ӧ�����ӷ���ʽΪAl(OH)3��OH��=Al[(OH)4]����Al(OH)3��OH��=AlO2����2H2O��
��4��BC�ζ�Ӧ�����ӷ���ʽAl(OH)3��OH��=AlO2����2H2O�����ĵ�����������0.001mol�����Ժ�����������0.001mol������þ���Ӻ�������һ��������������0.005mol������þ�������ʵ���Ҳ��0.001mol��A��õ��Ĺ�����������þ0.001mol����������0.001mol��������0.001mol��58g/mol+0.001mol��78g/mol=0.136g��
��5������Һ�д��ڵ���������NO3-������ͼ��ʼ�����������к������ӣ�����Һ������������0.001mol��AB������������笠���Ӧ���������������笠�������0.002mol�������ӡ�þ���Ӹ���0.001mol�����ݵ���غ㣬������������ʵ���Ϊ0.001mol+0.002mol+0.002mol+0.003mol��0.008mol��Ũ����0.008mol��0.1L��0.08mol/L��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��̽��FeSO4��Cu(NO3)2�Ļ�����и���ֵĺ�����������������̣�
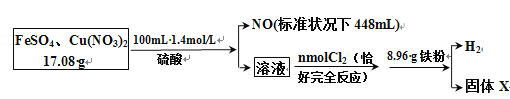
ͨ������ó�:
(1)ԭ�������FeSO4����������ԼΪ_______��
(2)ͨ��� n(Cl2) =________ mol��
(3)���ɵ�H2�ڱ�״���µ������________mL��
(4)����X������________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͼ����H2O2ø������pHӰ������ߣ�ͼ�ұ�ʾ�������¶��¡�pH=bʱH2O2�ֽ������O2����ʱ��ı仯������ø�ٷ�Ӧ�����иı�ijһ��ʼ���������¸ı���ȷ���ǣ� ��
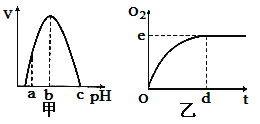
A��pH=aʱ��e�����ƣ�d������
B��H2O2ø������ʱ��e�����ƣ�d������
C��H2O2������ʱ��e�㲻�ƣ�d������
D���¶�����ʱ��e�㲻�ƣ�d������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾװ�ý�������ʵ�飺��������Һ�������У�Ԥ���������ʵ���������

ѡ�� | �������� | �������� | Ԥ�����е����� |
A�� | ϡ���� | ̼�������������ƵĻ����Һ | ������������ |
B�� | Ũ���� | ��ɰֽ��ĥ�������� | ��������ɫ���� |
C�� | �Ȼ�����Һ | Ũ����������Һ | ����������ɫ���� |
D�� | ������Һ | �������������Һ | ��Һ����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ����������������ء��밴Ҫ�ش��������⣺
(1)���˽����������������α������������Ƴɣ�������ijɷֿɼ����� Ca2Mg5Si8O22(OH)2�����������������ʽ�ɱ�ʾΪ___________________��
(2)�й�Na2CO3��NaHCO3 ����;������������ͷ۵���Ҫ�ɷ�֮һ�ǣ�д�׳ƣ�________��д�����������У�������������θ������ҩ����θ�ᷴӦ�����ӷ���ʽ____________��
(3)��Ư�������ڱ�¶�ڿ����л���ʣ�������Ϊ��Ư����������ж�����̼�Ӵ����Ӷ�ʧȥ��Ư���ԡ�д���ò���Ӧ�Ļ�ѧ��Ӧ����ʽ___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ģ����и�ѡ���йض�Ӧ�л����˵����ȷ����(����)
A |
| �����18��ԭ�Ӵ���ͬһƽ���� |
B |
| �����ϵ�̼ԭ������5 |
C |
| �������Եõ�3�������� |
D |
| ��������1��3�� 4�������� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵������ȷ����
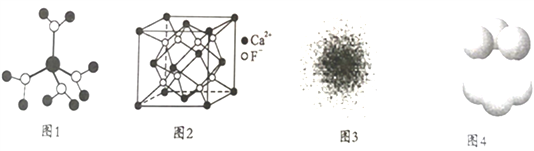
A. ˮ��ͭ���ӵ�ģ����ͼ1��ʾ��1��ˮ��ͭ��������4����λ��
B. CaF2����ľ�����ͼ2��ʾ��ÿ��CaF2����ƽ��ռ��4��Ca2+
C. ��ԭ�ӵĵ�����ͼ��ͼ3��ʾ����ԭ�Ӻ���Ĵ����������ԭ�Ӻ˸����˶�
D. ����Cu��ͭԭ�Ӷѻ�ģ����ͼ4��ʾ���ý�������Ϊ���ܶѻ���ÿ��ͭԭ�ӵ���λ����Ϊ12
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С����̽��SO2�Ļ�ѧ���ʣ����������ʵ�鷽����
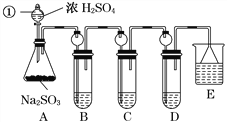
(1)ָ�������������ƣ�______________��
(2)���Aװ�õ������Եķ�����______________________________________________��
(3)װ��B����SO2�������ԣ���B����ʢ�Լ�����Ϊ________��
(4)װ��C��ʢװ��ˮ���Լ���SO2��________�ԣ���C�з�Ӧ�����ӷ���ʽΪ
________________________________________________________________________��
(5)װ��D��ʢװ����Ư��Ũ��Һ��ͨ��SO2һ��ʱ���D�г����˴�����ɫ������ͬѧ�Ƕ�ɫ�����ɷ�������ּ��裺
������һ���ð�ɫ����ΪCaSO3��
��������ð�ɫ����Ϊ__________________________________________________��
���������ð�ɫ����Ϊ�����������ʵĻ���
�����ڼ���һ��ͬѧ�Ƕ�ɫ�����ɷֽ�����̽����������·�����
��ѡ���������Լ�������װ�á��Թܡ��ιܡ������ܵĵ�����������ˮ��0.5 mol��L��1HCl��0.5 mol��L��1H2SO4��0.5 mol��L��1BaCl2��1 mol��L��1NaOH��Ʒ����Һ��
��1������D�г������ˡ�ϴ�Ӹɾ������á�
��ش�ϴ�ӳ����ķ�����____________________________________________________��
��2��������һֻ�ɾ��Թ�ȡ����������Ʒ������________(�Լ�)�����ϴ����ܵĵ������������ܵ���һ�˲���ʢ��________(�Լ�)���Թ��С�������__________________���������һ������
�����������������д�����ɸð�ɫ�����Ļ�ѧ����ʽ��_________________________________��
(6)װ��E��ʢ�ŵ��Լ���________��������__________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com