
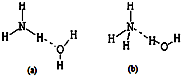
 �±���Ԫ�����ڱ���һ���֣�����������ĸ�ֱ����ijһ��ѧԪ�أ�
�±���Ԫ�����ڱ���һ���֣�����������ĸ�ֱ����ijһ��ѧԪ�أ�| a | b | ||||||||||||||||
| c | d | ||||||||||||||||
| e | f | g | h | i | |||||||||||||
| j | |||||||||||||||||
| � | X | Y | |
| ʧȥ��һ������ | 519 | 502 | 580 |
| ʧȥ�ڶ������� | 7296 | 4570 | 1820 |
| ʧȥ���������� | 11799 | 6920 | 2750 |
| ʧȥ���ĸ����� | 9550 | 11600 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ȶ��ԣ�H2S��HF |
| B����±������HF���ȶ� |
| C��һ��D2O����������������Ϊ8 |
| D��HCl�����ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
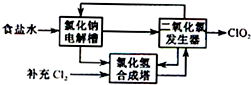 �������ȣ�ClO2����һ�ָ�Ч�����ס���ȫ��ɱ������������
�������ȣ�ClO2����һ�ָ�Ч�����ס���ȫ��ɱ�������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
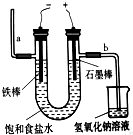
| A��ͨ��һ��ʱ��������������ĵ缫��Χ����Һ��ʹ��̪��� |
| B�������������ĵ缫������������ |
| C���븺�������ĵ缫������������ |
| D��Ϊ���������֮�䷢����Ӧ������ʯ��Ĥ�����ӽ���Ĥ�����۷ָ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
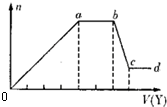
 ��ͼ����MgCl2��AlCl3�����Һ�У��Ⱥ�����Լ�A��Bʱ���ó������ʵ���y��mol�� ���Լ����V��mL����Ĺ�ϵͼ����ʼ�μ�6mL�Լ�A������μ�A���ɣ�֮��ĵ��Լ�B�����½�����ȷ���ǣ�������
��ͼ����MgCl2��AlCl3�����Һ�У��Ⱥ�����Լ�A��Bʱ���ó������ʵ���y��mol�� ���Լ����V��mL����Ĺ�ϵͼ����ʼ�μ�6mL�Լ�A������μ�A���ɣ�֮��ĵ��Լ�B�����½�����ȷ���ǣ�������| A��A������NaOH��B���������ᣬ��2cA=cB |
| B��ԭ���Һ�У�c��Al3+����c��Mg2+����c��Cl-��=1��2��7 |
| C��A������Ba��OH��2��B���������ᣬ��cA=2cB |
| D����A��B��ΪһԪǿ���һԪǿ���μ�7mL�Լ�A��ĵ��Լ�B |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | CO32-��SiO32-��AlO2-��Cl- |
| ������ | Al3+��Cu2+��Mg2+��NH4+��Na+ |
 ����Y�����ᣬ����Һ�к��еĽ�����������
����Y�����ᣬ����Һ�к��еĽ������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com