[ѡ��3-���ʽṹ������]
ԭ���������������X��Y��Z��G��Q��R��T����Ԫ�أ��˵������С��36����֪X�� һ��1��2���⻯������м��ЦҼ����Цм���������ԭ�ӹ�ƽ�棻Z��L������2��δ �ɶԵ��ӣ�Qԭ��s�ܼ���P�ܼ���������ȣ�R������������ּ���������Ӳ�Ʒ �ĺ��IJ��ϣ�T�������ڱ���ds����ԭ����ֻ��һ��δ�ɶԵ��ӣ�
��1��Yԭ�Ӻ����
�ֲ�ͬ�˶�״̬�ĵ��ӣ�Tԭ����
�ֲ�ͬ�ܼ��ĵ��ӣ�
��2��X��Y��Z�ĵ�һ��������С�����˳��Ϊ
����Ԫ�ط��ű�ʾ����
��3����X��Y��Z�γɵ�����ZXY
-��XZ
2��Ϊ�ȵ����壬��ZXY-��Xԭ�ӵ��ӻ������ ��Ϊ
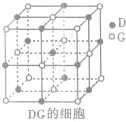
��4��Z��R���γɻ�����ף�1mol���к�
mol��ѧ������������ᷴӦ������ ��ķ��ӿռ乹�ͷֱ�Ϊ
��5��G��Q��R��������۵����±�������۵�����ԭ��Ϊ
| ������ |
G�ķ����� |
Q�ķ����� |
R�ķ����� |
�۵�/K |
993 |
1539 |
183 |
��6����T����������Һ����μ���Y���⻯���ˮ��Һ����������Ӧ�����ӷ���ʽΪ
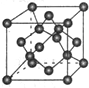
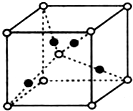
��7��X���ʵľ�����ͼ��ʾ��һ��X��������
��Xԭ�ӣ���X������ܶ�Ϊp g/cm
3�������ӵ�������ֵΪN
A����������� ������Xԭ��֮��ľ���Ϊ
cm���ô���ʽ��ʾ��


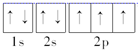 ��������ԭ�Ӻ����7�ֲ�ͬ�˶�״̬�ĵ��ӣ�Cuԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1������Cuԭ����7�ֲ�ͬ�ܼ��ĵ��ӣ�
��������ԭ�Ӻ����7�ֲ�ͬ�˶�״̬�ĵ��ӣ�Cuԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1������Cuԭ����7�ֲ�ͬ�ܼ��ĵ��ӣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
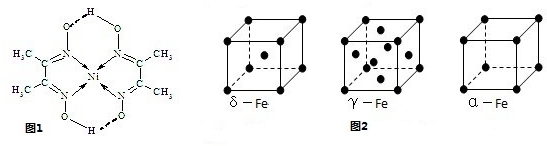
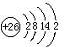
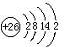
 ��3��H3+������һ�����������γɽṹ���ӵ�������[HG��A2C��5]2+�γɸ�������ʱ��H3+���ӽ����������ṩ��
��3��H3+������һ�����������γɽṹ���ӵ�������[HG��A2C��5]2+�γɸ�������ʱ��H3+���ӽ����������ṩ�� ����ѧ--ѡ��3���ʽṹ�����ʡ�
����ѧ--ѡ��3���ʽṹ�����ʡ�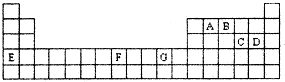
 ���������к��еĻ�ѧ��������
���������к��еĻ�ѧ��������
 ����ѧһѡ��3���ʽṹ�����ʡ�
����ѧһѡ��3���ʽṹ�����ʡ�