
ЁОЬтФПЁПзЯВЫгыКЃДјРрЫЦЃЌЪЧвЛжжИЛКЌЩњЮяЕтЕФКЃбѓжВЮяЃЌЩЬЦЗзЯВЫЧсБЁЫЩДрЁЂБШКЃДјИќвзБЛБКЩеГЩЛвЃЈДЫЪБЕтзЊЛЏЮЊЕтЛЏЮяЮоЛњбЮЃЉЃЌгУгкЕтЕЅжЪЕФЬсШЁЃЌМКжЊ:
ввНЭ | ЫФТШЛЏЬМ | СбЛЏЦћгЭ | ЕтЃЈОЇЬхЃЉ | |
УмЖШgcm-3 | 0.7893 | 1.595 | 0.71~0.76 | 4.94 |
ЗаЕу/Ёц | 78.5 | 76.8 | 25~232 | 184.35 |
вдЯТЮЊФГаЫШЄаЁзщФЃФтДгзЯВЫЬсШЁЕтЕЅжЪЕФЙ§ГЬЃК
![]()
(1)ЪЕбщЪвБКЩезЯВЫЃЌашвЊЯТСавЧЦїжаЕФ___________ЃЈЬюађКХЃЉЁЃ
a.ЪдЙм b.ЩеБ c.лслі d.ФрШ§НЧ e.еєЗЂУѓ f.ОЦОЋЕЦ g.ШМЩеГз
(2)НЋБКЩеЫљЕУЕФзЯВЫЛвгызуСПЕФЫЋбѕЫЎКЭЯЁСђЫсзїгУЃЌаДГіЗДгІЕФРызгЗНГЬЪН___________ЁЃ
(3)ВйзїЂйЕФУћГЦЪЧ__________ЃЛЪдМСAЮЊ_________ (ЬюБОЬтБэИёжавЛжжзюМбЛЏбЇЪдМСЕФУћГЦЃЉЃЌВЛЪЙгУСэЭтСНжжЪдМСЕФжївЊдвђЗжБ№ЪЧЃКI_____________________ЃЛII __________________ЁЃ
(4)ВйзїЂкгІдк____________(вЧЦїУћГЦЃЉжаеёЕДЁЂОВжУЃЛЙлВьЕНЕФЯжЯѓЪЧ______________ЁЃ
(5)ИУЗНАИВЩгУГЃбЙМгШШеєСѓВЂВЛКЯРэЃЌРэгЩЪЧ____________________ЁЃ
ЁОД№АИЁП c d f 2I-+H2O2+2H+=I2+2H2O Й§ТЫ ЫФТШЛЏЬМ ввДМгыЫЎЛЅШм СбЛЏЦћгЭФмгыЕтЗЂЩњМгГЩЗДгІ ЗжвКТЉЖЗ ЩЯВуЮоЩЋЃЌЯТВузЯЩЋ ЕтЕЅжЪвзЩ§ЛЊЃЌЛсЕМжТЕтЕФЫ№ЪЇ
ЁОНтЮіЁПЃЈ1ЃЉБКЩезЯВЫЪБгУлсліЪЂЗХзЯВЫЃЌгУДјЬњШІЕФЬњМмЬЈЗХжУФрШ§НЧЃЌФрШ§НЧЩЯЗХжУлсліЃЌгУОЦОЋЕЦНјааМгШШЃЌЫљвдБКЩезЯВЫЪБашвЊгУЕНЕФЪЕбщвЧЦїЪЧлсліЁЂДјЬњШІЕФЬњМмЬЈЁЂОЦОЋЕЦЁЂФрШ§НЧЃЌМДcЁЂdЁЂfЃЛ
ЃЈ2ЃЉЫЋбѕЫЎОпгаЧПбѕЛЏадЃЌЫсадЬѕМўЯТЃЌЫЋбѕЫЎбѕЛЏЕтРызгЩњГЩЕтЕЅжЪЃЌздЩэБЛЛЙдЩњГЩЫЎЃЌЗДгІЗНГЬЪНЮЊH2O2+2I-+2H+=I2+2H2OЃЛ
ЃЈ3ЃЉЭЈЙ§ВйзїЂйЙ§ТЫЃЌЕУЕНВЛШмЕФВадќЃЌТЫвКЮЊЕтЕЅжЪШмвКЃЌРћгУгаЛњШмМСнЭШЁГіЕтЕЅжЪЃЌнЭШЁМСЕФбЁШЁБъзМЪЧЃКШмжЪдкнЭШЁМСжаЕФШмНтЖШДѓгкдкдШмМСжаЕФШмНтЖШЃЌШмжЪКЭнЭШЁМСВЛЗДгІЃЌнЭШЁМСКЭдРДШмМСВЛФмЛЅШмЃЌЬтИЩжаЬсЙЉЕФЫФжаЮяжЪЃЌввДМКЭЫЎФмЛЅШмЃЌВЛФмзінЭШЁМСЃЌСбЛЏЦћгЭКЌгаВЛБЅКЭЬМЬМЫЋМќФмгыЕтЗЂЩњМгГЩЗДгІЃЌВЛФмзінЭШЁМСЃЌЫФТШЛЏЬМЗћКЯнЭШЁМСЕФбЁШЁБъзМЃЌЫљвдПЩвдгУЫФТШЛЏЬМзїнЭШЁМСЃЛ
ЃЈ4ЃЉЃЉВйзїЂкЮЊнЭШЁЗжвКВйзїЃЌгУЕНЗжвКТЉЖЗЁЂЩеБЃЌЛьКЯвКгІдкЗжвКТЉЖЗжаеёЕДЁЂОВжУЃЌбЁдёЕФгаЛњЪдМСЫФТШЛЏЬМЕФУмЖШДѓгкЫЎЃЌЫљвдЫФТШЛЏЬМдкЫЎЕФЯТВуЃЌМДЙлВьЕНЩЯВуЮоЩЋЃЌЯТВузЯЩЋЃЛ
ЃЈ5ЃЉвђЕтЕЅжЪвзЩ§ЛЊЃЌЛсЕМжТЕтЕФЫ№ЪЇЃЌЙЪГЃбЙМгШШеєСѓВЛКЯРэЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЙигкгаЛњЮяЕФа№Ъіе§ШЗЕФЪЧ
A. ЫсадИпУЬЫсМиШмвКПЩвдМьбщГіБНКЭввДМЕФЛьКЯвКжаЕФввДМ
B. гыNaOHШмвКЗДгІЧвЗжзгЪНЮЊC2H4O2ЕФгаЛњЮявЛЖЈЪЧввЫс
C. БћЯЉЗжзгжаПЩФмга8ИідзгЙВДІгкЭЌвЛЦНУц
D. МзБНЕФвЛТШДњЮяга3жж
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЛЏбЇЮяжЪдкЩњЛюЁЂЩњВњЕФдЫгУУшЪіВЛе§ШЗЕФЪЧ
A. ЧтбѕЛЏФЦФмгыЖўбѕЛЏЙшЗДгІЃЌЙЪГЃгУЧтбѕЛЏФЦдкВЃСЇЩЯНјааПЬЛ
B. ЙЄвЕЩЯГЃгУАБЦјРДМьВщТШЦјЙмЕРЪЧЗёаЙТЉ
C. бѕЛЏТСЫзУћИегёЃЌФЭФЅЃЌГЃгУгкзіжсГаВФСЯ
D. ФЦКЭтЕФКЯН№ГЪвКЬЌЃЌГЃзіКЫЗДгІЖбЕФЕМШШМС
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЧќЮєЖрАЭ( )ПЩжЮСЦжБСЂадЕЭбЊбЙЫљжТЭЗЛшЁЂЭЗдЮКЭЗІСІЃЌХСН№ЩВЁЛМепЕФВНЬЌНЉжБЕШЁЃвдЯТЪЧЧќЮєЖрАЭЕФвЛжжКЯГЩТЗЯп(ЭЈГЃ
)ПЩжЮСЦжБСЂадЕЭбЊбЙЫљжТЭЗЛшЁЂЭЗдЮКЭЗІСІЃЌХСН№ЩВЁЛМепЕФВНЬЌНЉжБЕШЁЃвдЯТЪЧЧќЮєЖрАЭЕФвЛжжКЯГЩТЗЯп(ЭЈГЃ![]() МђаДЮЊBnClЃЌ
МђаДЮЊBnClЃЌ![]() МђаДЮЊCbzCl )ЃК
МђаДЮЊCbzCl )ЃК

ЛиД№ЯТСаЮЪЬтЃК
(1)ЗДгІЂйЕФЗДгІРраЭЮЊ_____________ЃЌЦфзїгУЮЊ_________________ЁЃ
(2)ЧќЮєЖрАЭжаЫљКЌЕФЗЧКЌбѕЙйФмЭХУћГЦЮЊ_______________ЃЌЧќЮєЖрАЭЗжзгжага___________ИіЪжадЬМдзгЁЃ
(3)ЗДгІЂкЮЊМгГЩЗДгІЃЌгаЛњЮяXЕФУћГЦЮЊ______________________ЁЃ
(4)![]() Яд________________адЃЈЬюЁАЫсЁБЁЂЁАжаЁБЛђЁАМюЁБЃЉЃЌаДГіЦфгыбЮЫсЗДгІЕФЛЏбЇЗНГЬЪНЃК_______________________ЁЃ
Яд________________адЃЈЬюЁАЫсЁБЁЂЁАжаЁБЛђЁАМюЁБЃЉЃЌаДГіЦфгыбЮЫсЗДгІЕФЛЏбЇЗНГЬЪНЃК_______________________ЁЃ
(5)![]() ЕФЭЌЗжвьЙЙЬхжаЃЌФмгыNaHCO3ШмвКЗДгІЩњГЩCO2ЕФЖўШЁДњЗМЯуЛЏКЯЮяга_______жжЃЌЦфжаКЫДХЙВеёЧтЦзЮЊЫФзщЗхЕФНсЙЙМђЪНЮЊ______________(ШЮаДвЛжж)ЁЃ
ЕФЭЌЗжвьЙЙЬхжаЃЌФмгыNaHCO3ШмвКЗДгІЩњГЩCO2ЕФЖўШЁДњЗМЯуЛЏКЯЮяга_______жжЃЌЦфжаКЫДХЙВеёЧтЦзЮЊЫФзщЗхЕФНсЙЙМђЪНЮЊ______________(ШЮаДвЛжж)ЁЃ
(6)ВЮееЩЯЪіКЯГЩТЗЯпЃЌвдЖдєЧЛљБНМзШЉЮЊдСЯ(ЮоЛњЪдМСШЮбЁ)ЃЌЩшМЦжЦБИЖдєЧЛљБНМзЫсЕФКЯГЩТЗЯпЃК______________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП25ЁцЪБЃЌ0.1 mol Na2CO3гыЯЁбЮЫсЛьКЯЫљЕУЕФЬхЛ§ЮЊ1 LЕФШмвКЃЌШмвКжаВПЗжЮЂСЃгыpH ЕФЙиЯЕШчЭМЫљЪОЁЃЯТСагаЙиШмвКжаРызгХЈЖШЙиЯЕа№Ъіе§ШЗЕФЪЧ
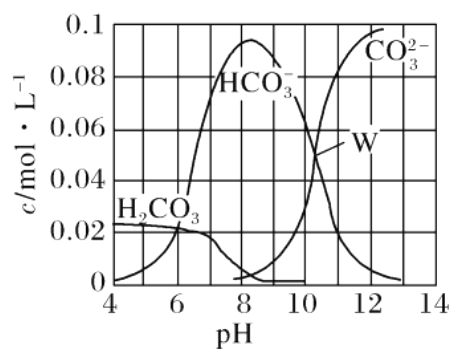
A. WЕуЫљЪОЕФШмвКЃКc(NaЃЋ)>c(CO![]() )ЃНc(HCO
)ЃНc(HCO![]() )>c(OHЃ)>c(HЃЋ)
)>c(OHЃ)>c(HЃЋ)
B. pHЃН4ЕФШмвКЃКc(H2CO3)ЃЋc(HCO![]() )ЃЋc(CO
)ЃЋc(CO![]() )ЃН0.1 molЁЄLЃ1
)ЃН0.1 molЁЄLЃ1
C. ЯђpHЃН8ЕФШмвКжаЭЈШыCO2жСpHЃН7ЫљЕУЕФШмвКЃКc(NaЃЋ)>c(ClЃ)ЃЋc(HCO![]() )ЃЋc(H2CO3)
)ЃЋc(H2CO3)
D. pHЃН11ЕФШмвКЃКc(NaЃЋ)ЃЋ2c(H2CO3)>2c(ClЃ)ЃЋ2c(CO![]() )
)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊЃК
ЂйCO(g) +H2O(g)=CO2(g)+H2(g) ЁїH = -41.2 kJ mol-1
ЂкCH4(g) +H2O(g)=CO(g) +3H2(g)ЕФФмСПБфЛЏШчЯТЭМЫљЪО

ЯТСагаЙиЫЕЗЈе§ШЗЕФЪЧ
A. ЂйКЭЂкОљЮЊЗХШШЗДгІ
B. ЂйЕФЗДгІЮязмФмСПЕЭгкЩњГЩЮязмФмСП
C. CO2(g) +CH4(g)=2CO(g) +2H2(g) ЁїW = -247.4 kJ mol-1
D. CH4(g) +H2O(g)=CO(g) +3H2(g)ЕФЛюЛЏФмДѓгк206.2 kJ mol-1
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПХфжЦ400 mL 0.5 molЁЄLЃ1ЕФNaOHШмвКЃЌЪдЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉМЦЫуЃКашвЊNaOHЙЬЬхЕФжЪСПЮЊ______ЁЃ
ЃЈ2ЃЉФГбЇЩњгУЭаХЬЬьЦНГЦСПвЛИіаЁЩеБЕФжЪСПЃЌГЦСПЧААбгЮТыЗХдкБъГпЕФСуПЬЖШДІЃЌЬьЦНОВжЙЪБЗЂЯж жИеыдкЗжЖШХЬЕФЦЋгвЮЛжУЃЌДЫЪБзѓБпЕФЭаХЬНЋ______(ЬюЁАИпгкЁБЛђЁАЕЭгкЁБ)гвБпЕФЭаХЬЁЃгћЪЙЬьЦНЦНКтЃЌЫљНјааЕФВйзїЮЊ_______ЁЃМйЖЈзюжеГЦЕУаЁЩеБЕФжЪСПЮЊ______(ЬюЁА32.6 gЁБЛђЁА31.61 gЁБ)ЃЌ
ЃЈ3ЃЉХфжЦЗНЗЈЃКЩшМЦЮхИіВйзїВНжшЃК
Ђй ЯђЪЂгаNaOHЕФЩеБжаМгШы200 mLеєСѓЫЎЪЙЦфШмНтЃЌВЂРфШДжСЪвЮТЃЛ
Ђк МЬајЭљШнСПЦПжаМгеєСѓЫЎжСвКУцНгНќПЬЖШЯп1ЁЋ2 cmДІЃЛ
Ђл НЋNaOHШмвКбиВЃСЇАєзЂШы500 mLШнСПЦПжаЃЛ
Ђм дкЩеБжаМгШыЩйСПЕФеєСѓЫЎЃЌаЁаФЯДЕг2ЁЋ3ДЮКѓвЦШыШнСПЦПЃЛ
Ђн ИФгУНКЭЗЕЮЙмМгеєСѓЫЎжСПЬЖШЯпЃЌМгИЧвЁдШЁЃ
ЪдНЋвдЩЯВйзїХХГіЯШКѓЫГађ______ЁЃ
ЃЈ4ЃЉФГбЇЩњЪЕМЪХфжЦNaOHШмвКЕФХЈЖШЮЊ0.48 molЁЄLЃ1ЃЌдвђПЩФмЪЧ______ЁЃ
AЃЎЪЙгУТЫжНГЦСПЧтбѕЛЏФЦЙЬЬх |
BЃЎШнСПЦПжадРДДцгаЩйСПеєСѓЫЎ |
CЃЎШмНтNaOHЕФЩеБЮДОЖрДЮЯДЕг |
DЃЎНКЭЗЕЮЙмМгЫЎКѓЖЈШнЪБбіЪгПЬЖШ |
ЃЈ5ЃЉдкЯТСаХфжЦ0.5 molЁЄLЃ1NaOHШмвКЙ§ГЬЪОвтЭМжагаДэЮѓЕФЪЧ(ЬюађКХ)______ЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГдЊЫиRзюИпМлКЌбѕЫсЕФЛЏбЇЪНЮЊHaRObЃЌдђЦфзюЕЭМлЦјЬЌЧтЛЏЮяжаRдЊЫиЕФЛЏКЯМл(ЁЁЁЁ)
A. 2b-a B. a-2b C. 8+a-2b D. 2b-a-8
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЪГДзЪЧШеГЃвћЪГжаЕФвЛжжЕїЮЖМСЃЌЙњМвБъзМЙцЖЈФ№дьЪГДзжаДзЫсКЌСПВЛЕУЕЭгк3.5 g/100 mLЁЃгУжаКЭЕЮЖЈЕФЗНЗЈПЩвдВтЖЈЪГДзжаДзЫсЕФХЈЖШЃЌФГАзДзЕФДзЫсХЈЖШВтЖЈЙ§ГЬШчЯТЭМЫљЪОЃК

![]()
ЭъГЩЯТСаЬюПеЃК
ЃЈ1ЃЉЯЁЪЭАзДзЪБашвЊЕФвЧЦїгаЩеБЁЂ_____________________________ЁЃ
ЃЈ2ЃЉгІбЁгУ__________зїЮЊжИЪОМСЁЃДяЕНЕЮЖЈжеЕуЪБЃЌжИЪОМСДг____ЩЋБфЮЊ_____ЩЋЁЃ
ЃЈ3ЃЉФГЭЌбЇвЛЙВНјааСЫШ§ДЮЪЕбщЁЃвдЯТЪЧЫћЩшМЦЕФЪЕбщЪ§ОнМЧТМБэЃЌБэИёжаAЪЧ______________ЃЌBЪЧ_______________ЁЃ
ЪЕбщДЮЪ§ | ЯЁЪЭКѓАзДз ЬхЛ§ЃЈmLЃЉ | БъзМNaOHШмвК | ||
A | B | ЯћКФЬхЛ§ЃЈmLЃЉ | ||
1 | 20.00 | 22.05 | ||
2 | 20.00 | 21.34 | ||
3 | 20.00 | 21.30 | ||
Ъ§ОнДІРэЃКЯћКФБъзМNaOHШмвКЕФЬхЛ§=______________mLЁЃ
ШєВтЕУЯЁЪЭКѓАзДзЕФХЈЖШ0.0594 mol/LЃЌдђИУЪГДз______ЃЈбЁЬюЁАЗћКЯЁБЁЂЁАВЛЗћКЯЁБЃЉЙњМвБъзМЁЃ
БъзМNaOHШмвКЭЈЙ§вдЯТВНжшзМБИЃКЂйХфжЦ500 mLХЈЖШдМЮЊ0.1 mol/LЕФNaOHШмвКЃЛ
ЂкгУKHC8H4O4БъзМШмвКзМШЗВтЖЈИУNaOHШмвКЕФХЈЖШЁЃ
ЃЈ4ЃЉГЦСПЫљашЕФNaOHЙЬЬхжУгкДѓЩеБжаЃЌМгШы500 mLеєСѓЫЎЃЌНСАшШмНтЃЌИУХфжЦВНжш____________
ЃЈЬюЁАПЩааЁБЛђЁАВЛПЩааЁБЃЉЁЃ
ЃЈ5ЃЉNaOHБъзМШмвКЕФХЈЖШашЭЈЙ§ВтЖЈЖјВЛФмжБНгХфжЦЕФдвђЪЧ__________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com