
���о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�____________________________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������____________________________________________________________________��
д��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ
____________________________________________________________________��
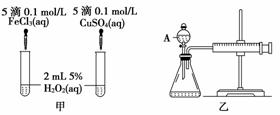
(2) ������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ________��ʵ������Ҫ������������__________________________________________________________________��
�����װ�������Եķ�����_____________________________________________________________________
____________________________________________________________________��
(3) 0.6 mol X�����0.6 mol Y��������2 L�ܱ������У��������·�Ӧ��
2X(g)��Y(g)===nZ(g)��2W(g)�� 2 minĩ����0.2 mol W���������Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol��(L��min)����ǰ2 min�ڣ���X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��2 minĩʱY�����ʵ���Ũ��Ϊ________����ѧ����ʽ�У�Z�Ļ�ѧ������n��________��
(4) ��һ���¶��£���Ӧ��2A(s)��2B(g) ![]() C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
A�������ڵ�ѹǿ����ʱ����仯
B����������ܶȲ�����ʱ����仯
C��A���������ٸı�
D��ƽ���������ƽ����Է����������ٸı�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)���о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�____________________________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������____________________________________________________________________��
д��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ
____________________________________________________________________��
(2) ������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ________��ʵ������Ҫ������������___________________________________________________________��
�����װ�������Եķ�����
____________________________________________________________________��
(3) 0.6mol X�����0.6mol Y��������2 L�ܱ������У��������·�Ӧ��
2X(g)��Y(g)===nZ(g)��2W(g)�� 2 minĩ����0.2 mol W���������Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol��(L��min)����ǰ2 min�ڣ���X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��2 minĩʱY�����ʵ���Ũ��Ϊ________����ѧ����ʽ�У�Z�Ļ�ѧ������n��________��
(4) ��һ���¶��£���Ӧ��2A(s)��2B(g) C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
A�������ڵ�ѹǿ����ʱ����仯
B����������ܶȲ�����ʱ����仯
C��A���������ٸı�
D��ƽ���������ƽ����Է����������ٸı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ������ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��7�֣����о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�________________________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������__________________________________��д��H2O2��MnO2�����·�����Ӧ�Ļ�ѧ����ʽ��______________________________��
(2) ������������ͼ����ʾ��ʵ��ʱ��������40 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ__________��ʵ������Ҫ������������_ _______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꽭��ʡ�ϲ�����У�����߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij������ȤС����ʵ��ķ�������̽����
��1��ʵ��һ��ȡ�����ʵ���Ũ�ȵ����H2O2��Һ�ֱ��������ʵ�飬ʵ�鱨�����±���ʾ��
|
��� |
���� |
���� |
���� |
|
|
�¶�/�� |
���� |
|||
|
1 |
40 |
FeCl3��Һ |
|
|
|
2 |
20 |
FeCl3��Һ |
|
|
|
3 |
20 |
MnO2 |
|
|
|
4 |
20 |
�� |
|
|
�Իش�ʵ��1��2�о���������������ͬʱ ��H2O2�ֽ����ʵ�Ӱ�졣
��2��ʵ��������о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч������С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
�� ���Է�������ͼ��ͨ���۲�________________________________�����ԱȽϵó����ۡ�
��ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������__________________________��
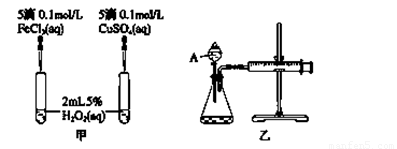
�ڶ���������Ϊ�˸���ȷ���о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죬������ͼװ�ý��ж���ʵ�顣��ɸ�ʵ��Ӧ�òⶨ��ʵ��������____________________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�ϲ��и߶���һ���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij������ȤС����ʵ��ķ�������̽����
��1��ʵ��һ��ȡ�����ʵ���Ũ�ȵ����H2O2��Һ�ֱ��������ʵ�飬ʵ�鱨�����±���ʾ��
|
��� |
���� |
���� |
���� |
|
|
�¶�/�� |
���� |
|||
|
1 |
40 |
FeCl3��Һ |
|
|
|
2 |
20 |
FeCl3��Һ |
|
|
|
3 |
20 |
MnO2 |
|
|
|
4 |
20 |
�� |
|
|
�Իش�
��ʵ��1��2�о����� ��H2O2�ֽ����ʵ�Ӱ�졣
��ʵ��2��3��Ŀ���� ��
��2��ʵ��������о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч������С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
�� ���Է�������ͼ��ͨ���۲�________________________________�����ԱȽϵó����ۡ�
��ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������__________________________��
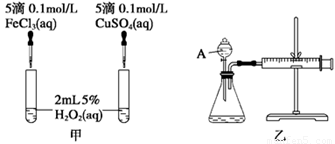
�ڶ�����������ͼ����ʾ��ʵ��ʱ��������40 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ʵ������Ҫ������������ ��
��3��ʵ��������֪2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+8H2O+10CO2�����ڸ������������Һ�Ͳ�����Һ��Ӧʱ�����ֿ�ʼһ��ʱ�䣬��Ӧ���ʽ�������Һ��ɫ�����Ե�����ͻȻ��ɫ����Ӧ�������Լӿ졣
���������ʵ������ijͬѧ��ΪKMnO4��H2C2O4��Ӧ�Ƿ��ȷ�Ӧ��������Һ�¶����ߣ���Ӧ���ʼӿ졣��Ӱ�컯ѧ��Ӧ���ʵ����ؿ�����IJ��뻹������_____��Ӱ�졣
������ʵ��֤����IJ��룬�����Ը��������Һ��������Һ�Լ��⣬����Ҫѡ����Լ���������� ��
A������� B�������� C��ˮ D���Ȼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�߶���ѧ������ģ��ѧ���϶����Ի�ѧ�Ծ� ���ͣ�ʵ����
(10��)���о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�____________________________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������____________________________________________________________________��
д��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ
____________________________________________________________________��
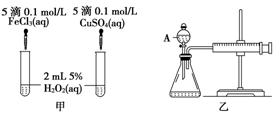
(2) ������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ________��ʵ������Ҫ������������___________________________________________________________��
�����װ�������Եķ�����
____________________________________________________________________��
(3) 0.6 mol X�����0.6 mol Y��������2 L�ܱ������У��������·�Ӧ��
2X(g)��Y(g)===nZ(g)��2W(g)�� 2 minĩ����0.2 mol W���������Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol��(L��min)����ǰ2 min�ڣ���X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��2 minĩʱY�����ʵ���Ũ��Ϊ________����ѧ����ʽ�У�Z�Ļ�ѧ������n��________��
(4) ��һ���¶��£���Ӧ��2A(s)��2B(g) 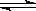 C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
A�������ڵ�ѹǿ����ʱ����仯
B����������ܶȲ�����ʱ����仯
C��A���������ٸı�
D��ƽ���������ƽ����Է����������ٸı�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com