
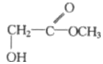
����Ŀ���л���A��B����Է���������С��200����ȫȼ��ʱֻ����CO2��H2O��Bȼ��ʱ���ĵ����������ɵĶ�����̼�����ʵ�����ȡ�B��̼����Ԫ���ܵ���������Ϊ46.67%��B������������Ӧ������NaHCO3��Һ��Ӧ�ų�CO2��1molAˮ������1mol�������1molB��A��Һ�������ԣ�����FeCl3��Һ����ɫ��
��1��A��B��Է�������֮��Ϊ___��
��2��B������Ӧ��___����ԭ�ӡ�
��3��A�Ľṹ��ʽΪ___��___��
��4��д��B��������������ͬ���칹��Ľṹ��ʽ___��___��___��___��
���𰸡�104 3 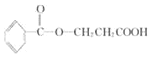
![]()
��������
����Bȼ��ʱ���ĵ����������ɵĶ�����̼�����ʵ�����ȣ���֪B��H��Oԭ�ӵĸ�����Ϊ2��1����B�ķ���ʽΪCxH2yOy����B��̼����Ԫ���ܵ���������Ϊ46.67%����ʽ ![]() ��x=y������B������������Ӧ������NaHCO3��Һ��Ӧ�ų�CO2����֪B�к����Ȼ�������ȩ������֪1molAˮ������1mol�������1molB����A+H20=C7H602+B��A��B�ķ�����С��200����֪B�ķ�����С��96�������ж�B�ķ���ʽΪC3H6O3���ٸ���A+H20=C7H602+B���ó�A�ķ�������
��x=y������B������������Ӧ������NaHCO3��Һ��Ӧ�ų�CO2����֪B�к����Ȼ�������ȩ������֪1molAˮ������1mol�������1molB����A+H20=C7H602+B��A��B�ķ�����С��200����֪B�ķ�����С��96�������ж�B�ķ���ʽΪC3H6O3���ٸ���A+H20=C7H602+B���ó�A�ķ�������
��1����Ϊ1molAˮ������1mol�������1molB����A+H20=C7H602+B����֪A��B��Է�������ֻ��Ϊ��122-18=104��
��2����ΪA��B����Է���������С��200����B�ķ�����С��96������Bȼ��ʱ���ĵ����������ɵĶ�����̼�����ʵ�����ȣ���֪B��H��Oԭ�ӵĸ�����Ϊ2��1����B�ķ���ʽΪCxH2yOy����B��̼����Ԫ���ܵ���������Ϊ46.67%����ʽ ![]() ��x=y���ƶ�B�ķ���ʽΪC3H6O3��B������Ӧ��3����ԭ�ӡ�
��x=y���ƶ�B�ķ���ʽΪC3H6O3��B������Ӧ��3����ԭ�ӡ�
��3������A+H20=C7H602+B������B�ķ�����Ϊ90����A�ķ�����Ϊ194�������к��б���������ʽΪC10H10O4����ΪA��Һ�������ԣ�����FeCl3��Һ����ɫ������ˮ�⣬������к����������Ȼ����������ǻ�����A�Ľṹ��ʽ����Ϊ![]() ��
��![]() ��
��
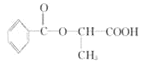
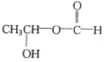
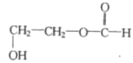
��4��B�ķ���ʽΪC3H6O3�������Ͷ�Ϊ1��B��������ͬ���칹���к������������������Ͷ�Ϊ1����2����ԭ�ӣ���B�п��ܻ������ǻ����Ѽ�����ͬ���칹���� ��
�� ��
�� ��
��![]() �ȡ�
�ȡ�


 �ŵ������ϵ�д�
�ŵ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���� NAΪ�����ӵ�������ֵ������˵���������
A.27 g ���������� 1mol/L �� NaOH ��Һ��ת�Ƶĵ�����Ϊ 3NA
B.18g ����(��ND2)�к��еĵ�����Ϊ 10NA
C.�� 100mL0.1mol/L ������Һ�м� CH3COONa ��������Һ�պ�Ϊ���ԣ���Һ�д��������Ϊ 0.01NA
D.�ö��Ե缫��� 100mL0.1mol/L �� CuSO4 ��Һ��������������������ͬ�����µ����������ʱ�� ��·��ת�Ƶ�����Ϊ 0.04NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ɵļ���ȼ�ϵ��ԭ����ͼ��ʾ������˵����ȷ����
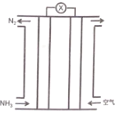
A.�������Һ�е�����������
B.��ظ�����ӦΪ��2NH3-6e-=N2+6H+
C.������ͨ�����������ͬ���������֮��Ϊ15: 4 (���������O2�������Ϊ20%)
D.�õ�ظ�Ǧ���س�磬ȼ�ϵ��������Ӧl molO2��Ǧ������2mol PbSO4������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��������A �� B ���������ֶ�����Ԫ����ɵ����ӻ���� ����������ͬ��A���������Ӹ�����Ϊ1 : 1�������������Ԫ����ɣ�������ܶ�Ϊ2.68gL-1 ���ҷ����и�ԭ��������������8 ���� ���Һͱ�Ϊ'�������壬����ʹ����ʯ��ˮ����ǣ�����ʹƷ����Һ��ɫ�����嶡��ʹʪ��ĺ�ɫʯ����ֽ������������A ���������̽���ʵ�顣
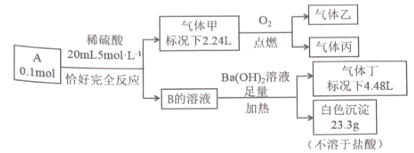
��ش�
(1)����ĵ���ʽ ______________��
(2)�����ͨ���������ᱵ��Һ�У�������Ӧ�����ӷ���ʽΪ____________��
(3)����A��ˮ��Һ�����������ӵ�ʵ�鷽��Ϊ_________________��
(4)������������һ�������� ________(�� �����ܡ� �����ܡ�) ����������ԭ��Ӧ����������д������Ϊ�����Ļ�ѧ����ʽ������������˵���������________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A.���ᱡ�ɴ���(![]() )���ܷ���ˮ�⡢��������ȥ��Ӧ
)���ܷ���ˮ�⡢��������ȥ��Ӧ
B.��ȩ�ͱ�ϩȩ(![]() )����ͬϵ�������������ַ�Ӧ��IJ���Ҳ����ͬϵ��
)����ͬϵ�������������ַ�Ӧ��IJ���Ҳ����ͬϵ��
C.��֪�����ۻ���Ϊ6.0kJ/mol�������������Ϊ20kJ/mol������ÿĦ��������2mol��������ۻ�����ȫ���ڴ��Ʊ�������������ֻ���ƻ�����15%�����
D.CH3COOCH2CH3��CH3CH2COOCH3��Ϊͬ���칹�壬1H��NMR����ʾ���߾������ֲ�ͬ����ԭ����������ԭ�ӵı�����ͬ���ʲ�����1H��NMR������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼��Ԫ�صĵ��ʺͻ�������зdz���Ҫ�����á���ش��������⡣
��1��̼��Ԫ����������������뵼����������__(��Ԫ������)����۵����Ų�ʽΪ___��
��2��CH3OH������Cԭ�ӵ��ӻ���ʽΪ__��SCN-�Ŀռ乹��Ϊ___��
��3��������(CnH2n+2)��n���������۷е����ߣ�ԭ����__��
�ڹ���̼ͬ�壬Ҳ��ϵ���⻯�������������������϶����٣�ԭ����__��
��4����ͼ��SiO2���������ɶ������辧��ṹ����С������__��ԭ�ӹ��ɡ���֪��������Ϊapm�����侧���ܶ�Ϊ__g��cm-3��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��100�����¶���(�����漰����Һ�¶Ⱦ�Ϊ100��)��ˮ�����ӻ�KW��1��10��12������˵����ȷ����
A.0.001 mol/L��NaOH��ҺpH��9
B.0.1 mol/L��H2SO4��ҺpH��1
C.0.005 mol/L��H2SO4��Һ��0.01 mol/L��NaOH��Һ�������ϣ������ҺpHΪ6����Һ������
D.��ȫ�к�pH��3��H2SO4��Һ50 mL����ҪpH��11��NaOH��Һ50 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ĺ������кܶ��֣������������ᡢ�������⣬���кܶ������ᣬ�罹������(H2S2O5)����һ����(H2SO5) ��������(H2S2O8) �ȡ���һ������һ��һԪǿ�ᣬ��������Ӿ����������ṹʽ��ͼ��ʾ������������һ�ְ�ɫ���壬�����ֽ⣬��ǿ��ˮ�ԣ���������ˮ����ˮ�л���ˮ��õ�����������⣬��ش�����������⡣
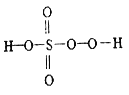
(1)��һ��������Ԫ�صĻ��ϼ���_________����������Ľṹʽ��_____________��
(2)��ҵ���Ʊ�����������Һ������֮һ���£�
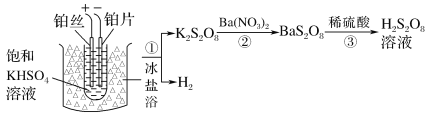
�ٵ��ʱ�����ĵ缫��ӦʽΪ______
�����������ܷ���ͭ˿���沬˿��________(������������������)��˵�����ɣ�__________��
(3)����������(Na2S2O5)����Ҫ�Ŀ�����������ҵ�������̵����е�SO2����Na2S2O5�Ĺ���Ϊ��
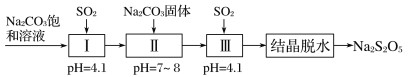
��pH��4.1ʱ������Ϊ________��Һ(д��ѧʽ)��
�ڹ����м���Na2CO3���壬���ٴγ���SO2��Ŀ����___________��
�����ѾƳ���Na2S2O5�������������ڲⶨij���Ѿ���Na2S2O5������ʱ��ȡ50.00 mL���Ѿ���Ʒ����0.010 00 mol��L��1�ĵ��Һ�ζ����յ㣬����10.00 mL���ζ���Ӧ�����ӷ���ʽΪ_______________ ������Ʒ��Na2S2O5�IJ�����Ϊ______g��L��1(��SO2��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I���ݱ������ҹ����Ϻ��������������еĿ�ȼ��(�����ˮ����)�Բɻ�óɹ���������һ����Ҫ�Ļ���ԭ�ϡ�
��1����������������������ʵ���Ҫ��ʽ����������������������֣�
ˮ����������CH4(g)��H2O(g) ![]() CO(g)��3H2(g)����H1����205.9 kJ��mol��1�� ��
CO(g)��3H2(g)����H1����205.9 kJ��mol��1�� ��
CO(g)��H2O(g) ![]() CO2(g)��H2(g)����H2����41.2 kJ��mol��1����
CO2(g)��H2(g)����H2����41.2 kJ��mol��1����
������̼������CH4(g)��CO2(g) ![]() 2CO(g)��2H2(g)����H3����
2CO(g)��2H2(g)����H3����
��Ӧ���Է����е�������______________����H3��________kJ��mol��1��
��.���Ĺ̶�һֱ�ǿ�ѧ���о�����Ҫ���⣬�ϳɰ������˹��̵��Ƚϳ���ļ�������ԭ��ΪN2 (g)��3H2 (g) ![]() 2NH3(g)��
2NH3(g)��
��2���ڲ�ͬ�¶ȡ�ѹǿ����ͬ���������£���ʼN2��H2 �ֱ�Ϊ0.1 mol��0.3 molʱ��ƽ��������а����������(��)����ͼ��ʾ��

�����У�p1��p2 ��p3 �ɴ�С��˳����____________���÷�Ӧ��H _______0(����>����<����������)��
�����ֱ���vA(N2)��vB(N2)��ʾ�ӷ�Ӧ��ʼ����ƽ��״̬A��Bʱ�Ļ�ѧ��Ӧ���ʣ���vA(N2)________vB(N2)(����>����<����������)��
������250 �桢p1 Ϊ105 Pa�����£���Ӧ�ﵽƽ��ʱ���������Ϊ1 L�����������B��N2 �ķ�ѹp(N2)Ϊ_______Pa (��ѹ����ѹ�����ʵ�������������һλС��)��
��.�����������(S2O42-)Ϊý�飬ʹ�ü�ӵ绯ѧ��Ҳ�ɴ���ȼú�����е�NO��װ����ͼ��ʾ��

��3�����������ĵ缫��ӦʽΪ___________��
��NO����ת�������Ҫ����ΪNH4+����ͨ��ʱ��·��ת����0.3 mol e�������ͨ����������������յ�NO�ڱ�״���µ����Ϊ________mL��
��.��4�������£���a mol��L-1�Ĵ�����b mol��L-1Ba(OH)2 ��Һ�������ϣ���ַ�Ӧ����Һ�д���2c(Ba2+)=c(CH3COO-)����û����Һ�д���ĵ��볣��Ka=___________(�ú�a��b�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com