
����Ŀ������VA��Ԫ���γɵĻ���������Ҫ�İ뵼����ϣ�Ӧ����㷺�����黯��(GaAs)���ش��������⣺
(1)��̬Gaԭ�ӵĺ�������Ų�ʽΪ_____����̬Asԭ�Ӻ�����_________��δ�ɶԵ��ӡ�
(2)��ʧȥ���ӵ�������(��λ��kJ��mol-1)����ֵ����Ϊ577��1984.5��2961.8��6192�ɴ˿���֪�ص���Ҫ���ϼ�Ϊ____��+3����ĵ縺�Ա���____(������������С��)��
(3)�Ƚ������ص�±������۵�ͷе㣬������仯���ɼ�ԭ��________________________��
�ص�±���� | GaCl3 | GaBr3 | GaI3 |
�۵�/�� | 77.75 | 122.3 | 211.5 |
�е�/�� | 201.2 | 279 | 346 |
GaF3���۵㳬��1000 �棬���ܵ�ԭ��___________________________________________��
(4)��ˮ�ϲ����صĽṹ��ͼ��ʾ��������ԭ�ӵ���λ��Ϊ______���������̼ԭ�ӵ��ӻ���ʽΪ______________��

(5)�黯���۵�Ϊ1238�棬���������ṹ��ͼ��ʾ����������Ϊa=565 pm���þ��������Ϊ_________��������ܶ�Ϊ___________(��NAΪ�����ӵ���������ֵ,�г���ʽ����)g��cm-3��

���𰸡�[Ar]3d104s24p1��1s22s22p63s23p63d104s24p1 3 +1 �� GaCl3��GaBr3��GaI3���۷е��������ߣ����Ǿ�Ϊ���Ӿ��壬�ṹ���ƣ���Է����������������Ӽ�������������ǿ GaF3Ϊ���Ӿ��� 4 sp2 ԭ�Ӿ��� ![]() g/cm3
g/cm3
��������
(1)Ga��ԭ������Ϊ31�����̬ԭ�ӵĵ���ʽ�Ų�ʽΪ��[Ar]3d104s24p1��1s22s22p63s23p63d104s24p1��As��ԭ������Ϊ33����As�Ļ�̬ԭ�ӵĵ����Ų�ʽΪ��[Ar]3d104s24p3�����Ի�̬Asԭ�Ӻ�����3��δ�ɶԵ��ӣ�
�ʴ�Ϊ��[Ar]3d104s24p1��1s22s22p63s23p63d104s24p1��3��
(2)����������̬ԭ��ʧȥ��������Ҫ�����������ص�ǰ�ļ������ܿ�֪������Ҫ���ϼ�Ϊ+1��+3������As�����������Ų�Ϊ4s24p3���ǰ����ȶ�״̬����Ga�����������Ų�Ϊ4s24p1�ر���4p1��ʧ���ӣ�����As�ĵ縺�Ա�Ga��
�ʴ�Ϊ��+1����
(3)�������ݿ�֪���ص�±������۵�ͷе㶼���ߣ��Ұ����ȡ��塢���������ߣ��������������ͬ���ṹ���ƣ����Ƿ��Ӿ��壬����������Է��������������Ӽ������������۷е����ߣ�GaF3���۵㳬��1000����������F�ĵ縺�Ժܴ��γɵ�GaF3�����Ӿ��壬
�ʴ�Ϊ��GaCl3��GaBr3��GaI3���۷е��������ߣ����Ǿ�Ϊ���Ӿ��壬�ṹ���ƣ���Է����������������Ӽ�������������ǿ��GaF3Ϊ���Ӿ��壻
(4)�ɶ�ˮ�ϲ����صĽṹͼ��֪����ԭ�ӵ���λ��Ϊ4���������̼ԭ�����Ȼ��е�̼ԭ�ӵ��ӻ���ʽ��ͬ���γɵĶ���ƽ��ṹ������Ӧ����sp2�ӻ���
�ʴ�Ϊ��4��sp2��
(5)���ڸþ�����۵�ߣ�������ض����ǻ���Ԫ�أ����Ըþ�����ԭ�Ӿ��壬�仯ѧʽΪGa4As4���þ��������m=![]() g�����ΪV=��565��10-10��3cm3�������ܶ�Ϊ��
g�����ΪV=��565��10-10��3cm3�������ܶ�Ϊ��![]() g/cm3��
g/cm3��
�ʴ�Ϊ��ԭ�Ӿ��壬![]() g/cm3��
g/cm3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���H��һ����Ҫ�ĸ߷��ӻ���������ϳ�·��������
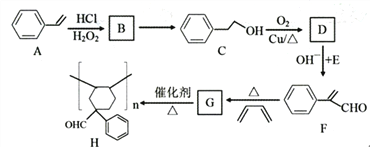
��֪��
��ش�����������
��1��A��������_______________��C���������������______________��
��2��д����Ӧ������A��B______________��C��D__________________��
��3��B��C�ķ�Ӧ�Լ��ͷ�Ӧ������______________________��
��4��D+E��F�ķ�Ӧ����ʽ��_________________��
��5��G�ķ���ʽ��____________________��
��6����������������F��ͬ���칹�干��__________��(�����������칹)��
a.������������ȡ��������������״�ṹ��b.��̼̼��������-C��COH�ṹ��
��7������������л��о�����Ҫ�������������![]() ��.CH3CHO��
��.CH3CHO��![]() -CHO�ϳɶ������
-CHO�ϳɶ������ ��·��(���Լ���ѡ)______________
��·��(���Լ���ѡ)______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������Ҫ��Ni������Al��Fe��SiO2�������������ᡢ������ʡ����÷��������Ʊ�NiSO4��7H2O���壬����������ͼ��

��֪��Ksp[Fe(OH)3]=8.0��10��38��Ka sp[Fe(OH)2]=8.0��10��16��K[Al(OH)3]=3.2��10��34��Ksp[Ni(OH)2]=2.0��10��15��1g2=0.3��
�ش���������
��1�����������Ŀ����______��
��2�������ʱ���������ӷ���ʽΪFe+2H+=Fe2++H2����_______��
��3��������������Ҫ�ȼ���H2O2��Һ��������Ӧ�����ӷ���ʽΪ______��Ȼ�����PH=_______ʱ��ʹ��������ǡ����ȫ����(ע������Ũ��С�ڻ����1��10��5mol/Lʱ������ȫ)
��4��������A��______��
��5��Ni2+��ǿ������Һ�л��ɱ�NaClO����ΪNiOOH���÷�Ӧ���ӷ���ʽΪ______��
��6�� NiOOHҲ����Ϊԭ��صĵ缫���ϣ����ڼ����������γ�ȼ�ϵ�أ�����ͨ��N2H4���壬���ĵ缫��ӦʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ӹ����������仯�������������������е����÷����˾�ı䡣
(1)�Ŵ��й��Ĵ���֮һ��ָ����������Ȼ��ʯ�Ƴɵ�,����Ҫ�ɷ���____(����ĸ���)��
a��Fe b��FeO c��Fe3O4 d��Fe2O3
(2)����������Ҫ��ѧ�ɷ�Ϊ��SiO2Լ45%��Fe2O3Լ40%��Al2O3Լ10%��MgOԼ5%���ø÷�����ȡҩ�ø��Ϻ��������Ĺ�����������(���ֲ�����������)��
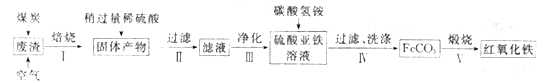
���ڲ�����в������ж����������_____________________��
���ڲ��������У�Ҫ��ȥ������֮һΪAl3+��������ʱKsp[Al(OH)3]=1.0��10-32����ʱ�����Ͻ�Al3+ ������ȫ������Һ��pHΪ_________��(c(Al3+)��1.0��10-5mol/L ��ΪAl3+������ȫ)
�۲�����У�����FeCO3�����ӷ���ʽ��____________________________��
(3)�Ȼ�����Һ��Ϊ��ѧ�Լ��е����������������Ȼ�ͭ���Ȼ����Ļ����Һ�м�������ͭ��ĩ�����������д���ó����Ļ�ѧʽ______________������ƽ���ƶ���ԭ������ϱ�Ҫ�����ӷ���ʽ���Դ������������ͣ�__________________________________________________________��
(4)�ٹ��϶��������ɫȾ����³ʿ���ĺϳɷ������£�

���ֽⷴӦ������ӷ���ʽ��____________________________________________��
����������³ʿ���ϳ�ԭ���ɼ��ʳƷ���Ƿ�CN-���������£�

����ֽ������֤��ʳƷ�к���CN-������ͼ��ʱ��ֽ��FeSO4�����ã�____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���⻯�ؿ������л��ϳɡ���ҩ�ȣ�����һ�ְ�ɫ�����ᾧ���ĩ��������ˮ��ʵ�����Ʊ�KI ��ʵ��װ������

ʵ�鲽�����£�
������ͼ��ʾ��C�м��� 127g ��ϸ�ĵ���I2 �� 195g 30%�� KOH ��Һ�����ҽ��衣
��I2��ȫ��Ӧ���ɼ�������ͨ�������� H2S��
�۽�װ�� C ��������Һ��ϡ H2SO4�ữ������ˮԡ�ϼ��� 10min��
����װ�� C ����Һ�м��� BaCO3 ����ֽ�����ˡ�ϴ�ӡ�
�ݽ���Һ��������ữ������Ũ����������ֽᾧĤ�� �� ��ϴ�ӡ����
�ò�Ʒ 145g��
�ش��������⣻
(1)����ٽ�����ϸ��Ŀ����_____________________________________��
(2)װ��A �з�����Ӧ�����ӷ���ʽΪ_______________________________ ��װ�� B �е��Լ���____________________��Cװ�õ�������_____________��
(3)װ�� C ��I2�� KOH ��Ӧ����֮һ�� KIO3 ���÷�Ӧ�Ļ�ѧ����ʽΪ_______________��
(4)����ܵ�Ŀ����___________________________________ ��
(5)������������� __________________��_______________��
(6)����ʵ�����Ϊ__________________ (������λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ĥ����ԭ���͵绯ѧԭ���Ʊ������������ɫ������N2O5��װ����ͼ��ʾ������˵����ȷ���ǣ� ��

A. �缫b��Ӧʽ��O2+4e-+2H2O=4OH-
B. ������װ��d�缫������Һ��pH����
C. c�缫�ϵĵ缫��ӦʽΪN2O4-2e-+H2O=N2O5+2H+
D. ����ÿ����1mol SO2����װ������1mol H+ͨ����Ĥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����11�֣���֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӡ�X��Y���γɻ�����X2Y3��ZԪ�ؿ����γɸ�һ�����ӡ���ش��������⣺
(1)XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ________����Ԫ�صķ�����________��
(2)YԪ��ԭ�ӵļ۲���ӵĵ����Ų�ͼΪ________����Ԫ�ص�������________��
(3)X��Z���γɻ�����XZ3���û�����Ŀռ乹��Ϊ________��
(4)��֪������X2Y3��ϡ������Һ�пɱ�����п��ԭΪXZ3�����ﻹ��ZnSO4��H2O���÷�Ӧ�Ļ�ѧ����ʽ��___________________________________________________________��
(5)�Ƚ�X���⻯����ͬ��ڶ���������Ԫ�����γɵ��⻯���ȶ��ԡ��е�ߵͲ�˵������
_________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������N2O5�����η����ֽⷴӦ����N2O5(g)![]() N2O3(g)��O2(g)����N2O3(g)
N2O3(g)��O2(g)����N2O3(g)![]() N2O(g)��O2(g)�������Ϊ2 L�ĺ����ܱ������г���8 mol N2O5�����ȵ�T ��ʱ��Ӧ�ﵽƽ��״̬����ʱO2��N2O3�����ʵ����ֱ�Ϊ9 mol��3.4 mol����T ��ʱ��Ӧ�ٵ�ƽ�ⳣ��Ϊ
N2O(g)��O2(g)�������Ϊ2 L�ĺ����ܱ������г���8 mol N2O5�����ȵ�T ��ʱ��Ӧ�ﵽƽ��״̬����ʱO2��N2O3�����ʵ����ֱ�Ϊ9 mol��3.4 mol����T ��ʱ��Ӧ�ٵ�ƽ�ⳣ��Ϊ
A. 10.7 B. 8.5 C. 9.6 D. 10.2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йشӺ�������ȡ���ʵ��ԭ����װ���ܴﵽʵ��Ŀ�ĵ���

A. ��װ�ü������麣��
B. ��װ���ҹ��˺����ҵĽ���Һ
C. ��װ�ñ��Ʊ�������������Һ��I��Cl2
D. ��װ�ö�������������Һ��I���Cl2β��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com