
����Ŀ���������庬���϶��Ԫ��֮һ���Ļ�������ҩ��������ũҩ����ȷ�����;�dz��㷺���ش��������⣺
��1����̬��ԭ�ӵĺ�������Ų�ʽΪ____________________��
��2��P4S3������������,����ӽṹ��ͼ����ʾ��
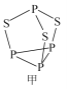

����һ����������_____________��;�縺������_____________��(�>����<��)��
��P4S3��������ԭ�ӵ��ӻ��������Ϊ_____________��
��ÿ��P4S3�����к��µ��ӶԵ���ĿΪ______________��
��3��N��P��As��Sb���ǵ�VA���Ԫ�ء�
������Ԫ�ص��⻯��ķе��ϵ��ͼ����ʾ���е㣺PH3<NH3,��ԭ����____________________;�е���PH3<AsH3<SbH3����ԭ����_____________________________________________________��
��ij�ִ��Ե������ľ����ṹ��ͼ����ʾ,�û�����Ļ�ѧʽΪ_________________��
��4�������۵�Ϊ2000�������뾧��軥Ϊ�ȵ����������������ṹ��ͼ����ʾ��
��ͼ��A���B���ԭ�����������ͼ����ʾ����C���ԭ���������Ϊ_______________��
������������ܶ�Ϊ��g��cm-3��NA��ʾ�����ӵ���������ֵ����þ����о��������������ԭ��֮��ľ���Ϊ_____________________________cm��
���𰸡� 1s22s22p63s23p3��[Ne]3s23p3 > < sp3 10 NH3���Ӽ���ڷ��Ӽ���� ��Է����������������Ӽ�������������ǿ Fe3N (![]() ��
��![]() ��
��![]() )
) ![]()
��������������������⿼�����ʽṹ�����ʣ��漰ԭ�Ӻ�������Ų�ʽ����д����һ�����ܺ͵縺�ԵıȽϣ�ԭ���ӻ���ʽ���жϣ����ʷе�ߵ͵ıȽϣ����廯ѧʽ��ȷ���Լ������ļ��㡣
��1��P�ĺ˵����Ϊ15��Pԭ�Ӻ��������Ϊ15�����ݹ���ԭ������̬Pԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p3��[Ne] 3s23p3��
��2����Pԭ�ӵļ۵����Ų�ʽΪ3s23p3��Sԭ�ӵļ۵����Ų�ʽΪ3s23p4��Pԭ�ӵ�3p���ڰ�������ȶ�����һ�����ܣ�P![]() S���ǽ�������P
S���ǽ�������P![]() S���縺�ԣ�P
S���縺�ԣ�P![]() S��
S��
���ɽṹ֪��P4S3��ÿ��Sԭ���γ�2���Ҽ���Sԭ���ϻ���2�Թµ��Ӷԣ���Sԭ��Ϊsp3�ӻ���
���ɽṹ֪��ÿ��Pԭ���γ�3�Թ��õ��Ӷԣ�ÿ��Pԭ������1�Թµ��Ӷԣ�ÿ��Sԭ���γ�2�Թ��õ��Ӷԣ�ÿ��Sԭ������2�Թµ��Ӷ���ÿ��P4S3�к��еŵ��Ӷ���Ϊ4![]() 1+3
1+3![]() 2=10��
2=10��
��3�����е���PH3![]() NH3��ԭ���ǣ�NH3���Ӽ���������PH3���Ӽ䲻����������е���PH3<AsH3<SbH3��ԭ���ǣ�PH3��AsH3��SbH3�����ڷ��Ӿ��壬��Է����������������Ӽ�������������ǿ���е����ߡ�
NH3��ԭ���ǣ�NH3���Ӽ���������PH3���Ӽ䲻����������е���PH3<AsH3<SbH3��ԭ���ǣ�PH3��AsH3��SbH3�����ڷ��Ӿ��壬��Է����������������Ӽ�������������ǿ���е����ߡ�
��������̯������Fe��12![]() +2
+2![]() +3=6��Nȫ�ھ����ڲ���N��2��N��Fe����N��N��=6:2=3:1����ѧʽΪFe3N��
+3=6��Nȫ�ھ����ڲ���N��2��N��Fe����N��N��=6:2=3:1����ѧʽΪFe3N��
��4�������վ���ͼʾ������ϵ�Լ�A��B�����꣬ѡA��Ϊ���յ㣬�۲�C���ھ�����λ�ã���Խ���![]() ��������A��B�����������֪C������Ϊ��
��������A��B�����������֪C������Ϊ��![]() ��
�� ![]() ��
�� ![]() ����
����
��������̯������1��������Al��8![]() +6
+6![]() =4��P��4������Ļ�ѧʽΪAlP��1mol���������Ϊ58g���辧���ı߳�Ϊx���������Ϊx3�����g/cm3
=4��P��4������Ļ�ѧʽΪAlP��1mol���������Ϊ58g���辧���ı߳�Ϊx���������Ϊx3�����g/cm3![]() NA=58g�����x=
NA=58g�����x=![]() cm�������о��������������ԭ��֮��ľ���Ϊ
cm�������о��������������ԭ��֮��ľ���Ϊ![]() x=
x=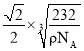 cm��
cm��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�������Һ�и�������һ���ܴ���������ǣ� ��
A.�����������������ɵ���Һ�У�Mg2+ �� Cl- �� NO3- �� K+
B.�����£�c(H+) =0.1 mol/L����Һ�У�Na+ �� AlO2-��S2-�� SO32-
C.����0.1 mol/LHCO3-����Һ��Na+ �� Fe3+ �� NO3- �� SCN-
D.![]() =0.1 mol/L����Һ��Na+ �� K+ �� CO32- �� NO3-
=0.1 mol/L����Һ��Na+ �� K+ �� CO32- �� NO3-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ�о���ѧϰС�����ԭ����γ������������ʵ�鷽������������̽����
(1)����д�й�ʵ�����ó���ؽ��ۡ�
��� | ʵ��װ�� | ʵ������ |
1 |
| п�����ܽ⣬�������������ɣ�ͭ������������ |
2 |
| ��п�����ܽ⣬��������������ɣ�������ָ�벻ƫת |
3 |
| ͭ�������������______________________��������ָ��___________________ |
��ͨ��ʵ��2��3���ɵó�ԭ��ص��γ�������______________________________��
��ͨ��ʵ��1��3���ɵó�ԭ��ص��γ�������______________________________��
������3װ�������ỻ���Ҵ���������ָ�뽫������ƫת���Ӷ��ɵó�ԭ����γ�������___________________��
(2)�ֱ�д��ʵ��3��Zn����Cu���Ϸ����ĵ缫��Ӧʽ��
Zn����______________________________��
Cu����______________________________��
(3)ʵ��3�ĵ����Ǵ�________������(�Zn����Cu��)����Ӧ����������0.4mol���ӷ�����ת�ƣ���Zn�缫��������___________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ������������ԭ��Ӧ���������ȷ�Ӧ���ǣ� ��
A.��ʯ����ˮ��������ʯ��
B.���ȵ�ľ̿��CO2��Ӧ
C.�����������е�ȼ�շ�Ӧ
D.Ba��OH��28H2O������NH4Cl����ķ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ȼ�ѧ����ʽ�Ц�H����ȼ���ȵ��� (����)
A. CH4(g)��![]() O2(g)===2H2O(l)��CO(g)����H1
O2(g)===2H2O(l)��CO(g)����H1
B. H2(g)��![]() O2(g)===H2O(g)����H2
O2(g)===H2O(g)����H2
C. C2H5OH(l)��3O2(g)===2CO2(g)��3H2O(l)����H3
D. 2CO(g)��O2(g)===2CO2(g)����H4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڲ�ͬ�¶��£�ˮ��Һ��c(H��)��c(OH-)��ϵ��ͼ��ʾ������˵������ȷ���ǣ� ��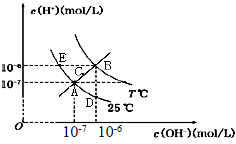
A.ͼ�����Kw��Ĺ�ϵ��B��C��A=D=E
B.E ���Ӧ��ˮ��Һ�У�������NH4+��Ba 2+��Cl����I������ͬʱ����
C.��0.1 mol/L ��NaHA ��Һˮ��Һ��c(H��)��c(OH��)��ϵ��ͼD ����ʾ������Һ���У�c(HA��)��c(OH��)��c(A2�� )��c(H2A)
D.��NaHSO4��Һ�е���Ba(OH)2��Һ����c(H��)��c(OH��)��ϵ��ͼA ����ʾ������Һ�з�Ӧ��2H��+SO42-+Ba2��+2OH��=BaSO4��+2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������һ����Ҫ�Ļ���ԭ�ϣ���ش���������:
I.�������(K2FeO4)��Һ���Ϻ�ɫ�������м�������������ƺ���Һ�Ϻ�ɫ����ȥ�������ֺ��ɫ��������д���÷�Ӧ�����ӷ���ʽ__________________��
I1.ʵ����ģ����ͼ��ʾ�����Ʊ���������:

��֪: �����������У����Ʒ�ӦҺ�¶���35~60����������Ҫ������Ӧ:
C6H12O6+ 12HNO3=3HOOC-COOH+9NO2��+3NO��+9H2O
������������Һ����NO ��NO2������Ӧ:
NO+NO2+2NaOH=2NaNO2+H2O��2NO2+2NaOH=NaNO3+NaNO2+H2O

��1��ľм����Ҫ�ɷ�����ά�أ������֪��Ϣ��,����Ϊ��ľм�м�ϡ�����������_____ �����������з�Ӧ�¶Ȳ��˸���60����ԭ����______________��
��2�������κδ��������˹��̽��У�����������Һ���պ����Һ�г���OH-������������ӣ�����һ����NO2-��NO2-����һ�������ӵ����ʵ���֮��Ϊ________��
��3��װ��B�����Ʊ�NaNO2��ʢ�b���Լ���NaOH(aq)�⣬��������_____ (����ĸ)��
A.NaCl(aq) B.Na2CO3(aq) C.NaNO3( aq )
��.�ⶨ��Ʒ����:
[ʵ�鲽��] ��ȷ����ag ��Ʒ���200mL��Һ
�ڴӲ�������Ƶ���Һ����ȡ20.00mL ������ƿ��
����c mol/L����KMnO4��Һ�ζ����յ�
���ظ����ϲ���3 �Σ���������KMnO4��Һ��ƽ�����ΪVmL
��1����ƿ�з�����Ӧ�����ӷ���ʽΪ____________��
��2����Ʒ��NaNO2 �Ĵ���Ϊ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ԭCO2�ǽ������ЧӦ����Դ�������Ҫ�ֶ�֮һ���о���������Cu/ZnO���������£���CO2��ͨ��H2�����߿ɷ�����������ƽ�з�Ӧ��
��Ӧ�� CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H1=-53.7 kJ��mol-1��
CH3OH(g)+H2O(g) ��H1=-53.7 kJ��mol-1��
��Ӧ�� CO2(g)+H2(g)![]() CO(g)+H2O(g)�� ����H2=+41.2 kJ��mol-1
CO(g)+H2O(g)�� ����H2=+41.2 kJ��mol-1
ijʵ���ҿ���һ����CO2��H2��ʼͶ�ϱ�������ͬѹǿ��,������ͬ��Ӧʱ��������ʵ�����ݣ����С��״�ѡ���ԡ���ָת����CO2�����ɼ״��İٷֱ�����
��Ӧ��� | T/K | ���� | CO2ת����/% | �״�ѡ����/% |
�� | 543 | Cu/ZnO���װ� | 12.3 | 42.3 |
�� | 543 | Cu/ZnO����Ƭ | 10.9 | 72.7 |
�� | 553 | Cu/ZnO���װ� | 15.3 | 39.1 |
�� | 553 | Cu/ZnO����Ƭ | 12.0 | 71.6 |
��1��CO2�ĵ���ʽ��_____________��
��2����Ӧ����ƽ�ⳣ������ʽ��K=______��
��3���ԱȢٺ͢ۿɷ��֣�ͬ�����������£��¶����ߣ�CO2ת�������ߣ� ���״���ѡ����ȴ���ͣ�����ͼ״�ѡ���Խ��͵Ŀ���ԭ��_______________��
�ԱȢ١��ڿɷ��֣���ͬ���¶��£�����Cu/ZnO����ƬʹCO2ת���ʽ����� ���״���ѡ����ȴ��ߣ�����ͼ״���ѡ������ߵĿ���ԭ��____________��
��4�����������CO2ת��ΪCH3OHƽ��ת���ʵĴ�ʩ��____��
a��ʹ��Cu/ZnO���װ�������
b��ʹ��Cu/ZnO����Ƭ������
c���ͷ�Ӧ�¶�
d��Ͷ�ϱȲ���,���ӷ�Ӧ���Ũ��
e������CO2��H2�ij�ʼͶ�ϱ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com