
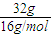 =2mol����ȫȼ������Һ̬ˮ���ų�1780.6kJ����������CH4ȼ�յ��Ȼ�ѧ����ʽΪCH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3kJ?mol-1
=2mol����ȫȼ������Һ̬ˮ���ų�1780.6kJ����������CH4ȼ�յ��Ȼ�ѧ����ʽΪCH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3kJ?mol-1

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ������ | X | Y | Z |
| �۵�/K | 1266 | 1534 | 183 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com