
| A�� | �ڢ� | B�� | �٢ۢݢ� | C�� | �ܢ� | D�� | �ڢܢޢ� |
���� ����c��NaOH��=$\frac{c�����ᣩV�����ᣩ}{V��NaOH��}$��������������V�����ᣩƫ�����²ⶨ���ƫ�ߣ��Դ������
��� �⣺����ʽ�ζ���������ˮϴ��δ�ñ�Һ��ϴ���൱��ϡ�������ᣬ����V�����ᣩƫ��c��NaOH��ƫ�ߣ��ʷ��ϣ�
�ڼ�ʽ�ζ���������ˮϴ��δ�ô���Һ��ϴ������ҺŨ��ƫС����ȡ����Һ�����ʵ���ƫС�����V�����ᣩƫС��c��NaOH���ͣ��ʲ����ϣ�
�۵ζ�ǰ��ʽ�ζ��ܼ��촦δ������Һ���ζ��յ������Һ�����V�����ᣩƫ��c��NaOH��ƫ�ߣ��ʷ��ϣ�
��ȡ��Һʱ�ζ��ܼ��촦δ������Һ��ȡ�������Һ������Һ�����ʵ���ƫС�����V�����ᣩƫС��c��NaOH���ͣ��ʲ����ϣ�
����ƿ������ˮϴ�����ô���Һ��ϴ������Һ�����ʵ���ƫ����V�����ᣩƫ��c��NaOH��ƫ�ߣ��ʷ��ϣ�
�ζ�ʱҡ����ƿʱ��Һ�彦��ƿ�⣬����Һ�����ʵ���ƫС�����V�����ᣩƫС��c��NaOH���ͣ��ʲ����ϣ�
�ߵζ������У��ζ���©��Һ�壬������ƿ�⣬����V�����ᣩƫ��c��NaOH��ƫ�ߣ��ʷ��ϣ�
���ȡ����Һ���ʱ���ζ�ǰ���ӣ��ζ����ӣ����V�����ᣩƫС��c��NaOH���ͣ��ʲ����ϣ�
ʹ���ƫ�ߵ��У��٢ۢݢߣ�
��ѡB��
���� ���⿼���к͵ζ���Ϊ��Ƶ���㣬��������к͵ζ���ʵ�ʡ�������Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע���Ϲ�ʽ��������Ŀ�ѶȲ���


 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
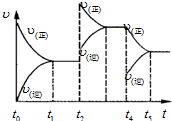 ��ҵ�������������Ҫ�������£�
��ҵ�������������Ҫ�������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH | B�� | Ba��NO3��2 | C�� | Na2CO3 | D�� | Ba��OH��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KCl | B�� | CuSO4 | C�� | HCl | D�� | H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaHSO4��Һ�м���Ba��OH��2��Һ��ǡ��������Ba2++OH-+H++SO42-�TBaSO4+H2O | |
| B�� | �����ʯ��ˮ��ͨ�����CO2��OH-+CO2�THCO3- | |
| C�� | ����������Һ��ϡ H2SO4 ��Ӧ��Ba2++SO42-�TBaSO4�� | |
| D�� | ̼��������ᷴӦ��CO32-+2H+�TH2O+CO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȡ����ˮʱ��Ϊ�˷�ֹƿ�ڲ�����������Ӧ������ƿ�ڼ��뼸Ƭ���Ƭ | |
| B�� | ϡ��Ũ����ʱ������ˮ���뵽ʢŨ������ձ��� | |
| C�� | ��CO��ԭFe2O3ʵ��ʱ��Ϊ��ֹCO��Ⱦ������ʵ����ϣ�����ֹͣͨCO����ֹͣ���� | |
| D�� | ��������ͭ��Һ��Ũ���ᾧʵ����Ҫ��������Ҫ���ձ����������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ˮ����ȡ�壬���þƾ�����ȡ�� | |
| B�� | ����ʵ����Ϻ���������ը�ѣ�������Ϊû�е�ʯ���� | |
| C�� | ��ȡ��ˮ��Һ�е⣬��������Ȼ�̼��Һʱ���۾�ע�ӷ�Һ©����Һ�� | |
| D�� | ����ʱ��Ϊ�˼ӿ�ˮ�����٣�ˮӦ���Ͽڽ��룬�¿����� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com