
¡¾̀âÄ¿¡¿ÏÂÁĐ˵·¨ƠưÈ·µÄÊÇ![]()
A.ÂóÑ¿̀ÇÓëƠá̀ǵÄË®½â²úÎï¾ùº¬ÆÏ̀Ñ̀Ç£¬¹Ê¶₫Ơß¾ùΪ»¹ÔĐͶ₫̀Ç
B.ỂÎÂÏ£¬½«![]() HAÈÜ̉ººÍ
HAÈÜ̉ººÍ![]() ÈÜ̉ºµÈ̀å»ư»́ºÏ
ÈÜ̉ºµÈ̀å»ư»́ºÏ![]() ºöÂỒå»ưµÄ±ä»¯
ºöÂỒå»ưµÄ±ä»¯![]() ²âµĂ»́ºÏÈÜ̉ºµÄ
²âµĂ»́ºÏÈÜ̉ºµÄ![]() £¬Ộ»́ºÏÈÜ̉ºÖĐÓÉË®µçÀë³öµÄ
£¬Ộ»́ºÏÈÜ̉ºÖĐÓÉË®µçÀë³öµÄ![]()
C.´¿̀¼Đ²ÄÁÏ¡°̀¼ÄÉĂ×ÅƯÄ¡±£¬Ă¿¸öÅƯĺ¬ÓĐÔ¼4000¸ö̀¼Ô×Ó£¬Ö±¾¶Ô¼6µ½9nm£¬ÔÚµÍÓÚ![]() ʱ£¬ÅƯľßÓĐÓÀ¾Ă´ÅĐÔ£¬¡°̀¼ÄÉĂ×ÅƯÄ¡±Óëʯī»¥ÎªÍ¬ËØ̉́ĐÎ̀å
ʱ£¬ÅƯľßÓĐÓÀ¾Ă´ÅĐÔ£¬¡°̀¼ÄÉĂ×ÅƯÄ¡±Óëʯī»¥ÎªÍ¬ËØ̉́ĐÎ̀å
D.̉ÑÖª![]() µÄ
µÄ![]() Ϊ
Ϊ![]() £¬Ộ½«µÈ̀å»ưµÄ
£¬Ộ½«µÈ̀å»ưµÄ![]()
![]() µÄ
µÄ![]() ÈÜ̉ººÍ
ÈÜ̉ººÍ![]()
![]()
![]() ÈÜ̉º»́ºÏºó»áÓĐ
ÈÜ̉º»́ºÏºó»áÓĐ![]() ³Áµí²úÉú
³Áµí²úÉú
¡¾´đ°¸¡¿C
¡¾½âÎö¡¿
A. Ơá̀ÇË®½âºóµÄ²úÎïÆÏ̀Ñ̀ÇÓĐ»¹ÔĐÔ£¬×ÔÉíĂ»ÓĐ»¹ÔĐÔ£¬Ëù̉Ô²»ÊÇ»¹ÔĐͶ₫̀Ç£¬¹ÊA´íÎó£»
B. ²âµĂ»́ºÏÈÜ̉ºµÄpH=5,HA¹ưÁ¿,ÈÜ̉ºÏÔʾËáĐÔ,·´Ó¦ºóµÄ»́ºÏ̉ºÖĐÇâÑơ¸ùÀë×ÓÊÇË®µçÀëµÄ,Ëù̉ÔË®µçÀëµÄÇâÀë×ÓŨ¶ÈΪ£º![]() £¬¹ÊB´íÎó£»
£¬¹ÊB´íÎó£»
C. ¡°̀¼ÄÉĂ×ÅƯÄ¡±ÊôÓÚ̀¼µ¥ÖÊÓëʯī»¥ÎªÍ¬ËØ̉́ĐÎ̀壬¹ÊCƠưÈ·£»
D. µÈ̀å»ư»́ºÏºó,![]() ,
,![]() ,
,![]() ¡£
¡£
¹ÊĂ»ÓĐ³ÁµíÎö³ö£¬¹ÊD´íÎó¡£
¹ÊÑ¡C.


 ̉»±¾ºẰâ¿ÚËằ⿨ϵÁĐ´đ°¸
̉»±¾ºẰâ¿ÚËằ⿨ϵÁĐ´đ°¸
| Ä꼶 | ¸ßÖĐ¿Î³̀ | Ä꼶 | ³ơÖĐ¿Î³̀ |
| ¸ß̉» | ¸ß̉»Ăâ·Ñ¿Î³̀ÍƼö£¡ | ³ở» | ³ở»Ăâ·Ñ¿Î³̀ÍƼö£¡ |
| ¸ß¶₫ | ¸ß¶₫Ăâ·Ñ¿Î³̀ÍƼö£¡ | ³ơ¶₫ | ³ơ¶₫Ăâ·Ñ¿Î³̀ÍƼö£¡ |
| ¸ßÈư | ¸ßÈưĂâ·Ñ¿Î³̀ÍƼö£¡ | ³ơÈư | ³ơÈưĂâ·Ñ¿Î³̀ÍƼö£¡ |
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
¡¾̀âÄ¿¡¿ÔÚ25 ¡æʱ£¬ÓĂʯīµç¼«µç½â2.0 L 0.5 mol¡¤L£1CuSO4ÈÜ̉º¡£5 minºó£¬ÔÚ̉»¸öʯīµç¼«ÉÏÓĐ6.4 gCuÉú³É¡£ÊԻشđÏÂÁĐÎỀ⣺
(1)·¢ÉúÑơ»¯·´Ó¦µÄ________¼«£¬µç¼«·´Ó¦Ê½Îª ____________________________
(2)·¢Éú»¹Ô·´Ó¦µÄ________¼«£¬µç¼«·´Ó¦Ê½Îª ____________________________
(3)Èô½«ÈÜ̉º»Ö¸´µ½Óëµç½âÇ°̉»Ñù£¬ỘĐè¼ÓÈë ________ molµÄ________________¡£
(4)ÈôÓõÈÖÊÁ¿µÄÁ½¿éÍƬ´ú̀æʯī×÷µç¼«£¬µç½âºóÁ½ÍƬµÄÖÊÁ¿Ïà²î ________________g£¬µç½ẩºµÄpH ________¡£(̀î¡°±äĐ¡¡±¡¢¡°±ä´ó¡±»̣¡°²»±ä¡±)
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
¡¾̀âÄ¿¡¿̉ÑÖªHCN(aq)ÓëNaOH(aq)·´Ó¦Éú³É1 molƠưÑεĦ¤H£½£12.1 kJ/mol£»Ç¿Ëᡢǿ¼îµÄÏ¡ÈÜ̉º·´Ó¦µÄÖĐºÍÈȦ¤H£½£57.3 kJ¡¤mol£1¡£ỘHCNÔÚË®ÈÜ̉ºÖеçÀëµÄ¦¤HµÈÓÚ£¨ £©
A. £69.4 kJ¡¤mol£1 B. £45.2 kJ¡¤mol£1
C. £«69.4 kJ¡¤mol£1 D. £«45.2 kJ¡¤mol£1
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
¡¾̀âÄ¿¡¿Èç±íÊDz»Í¬Î¶ÈÏÂË®µÄÀë×Ó»ưÊư¾Ư£º
ÎÂ¶È | 25 |
|
|
Ë®µÄÀë×Ó»ư³£Êư |
|
|
|
ÊԻشđ̉ÔÏÂÎỀ⣺
![]() Èô
Èô![]() £¬Ộ
£¬Ộ![]() __________
__________![]() ̀î¡°
̀î¡°![]() ¡±¡¢¡°
¡±¡¢¡°![]() ¡±»̣¡°
¡±»̣¡°![]() ¡±
¡±![]() £¬×÷³ö´ËÅĐ¶ÏµÄÀíÓÉÊÇ__________¡£
£¬×÷³ö´ËÅĐ¶ÏµÄÀíÓÉÊÇ__________¡£
![]() Ï£¬Ä³
Ï£¬Ä³![]() ÈÜ̉ºÖĐ
ÈÜ̉ºÖĐ![]() £¬È¡¸ĂÈÜ̉º
£¬È¡¸ĂÈÜ̉º![]() £¬¼ÓˮϡÊÍÖÁ
£¬¼ÓˮϡÊÍÖÁ![]() £¬ỘÏ¡ÊͺóÈÜ̉ºÖĐ
£¬ỘÏ¡ÊͺóÈÜ̉ºÖĐ![]() span>__________¡£
span>__________¡£
![]() Ï£¬½«
Ï£¬½«![]() µÄ¿ÁĐÔÄÆÈÜ̉º
µÄ¿ÁĐÔÄÆÈÜ̉º![]() Óë
Óë![]() µÄÏ¡Ạ́Ëá
µÄÏ¡Ạ́Ëá![]() »́ºÏ
»́ºÏ![]() Éè»́ºÏºóÈÜ̉ºµÄ̀å»ưΪÔÁ½ÈÜ̉º̀å»ưÖ®ºÍ
Éè»́ºÏºóÈÜ̉ºµÄ̀å»ưΪÔÁ½ÈÜ̉º̀å»ưÖ®ºÍ![]() £¬ËùµĂ»́ºÏÈÜ̉ºµÄ
£¬ËùµĂ»́ºÏÈÜ̉ºµÄ![]() £¬Ộ
£¬Ộ![]() __________¡£´ËÈÜ̉ºÖи÷ÖÖÀë×ÓµÄŨ¶ÈÓÉ´óµ½Đ¡µÄÅÅÁĐ˳Đ̣ÊÇ__________________¡£
__________¡£´ËÈÜ̉ºÖи÷ÖÖÀë×ÓµÄŨ¶ÈÓÉ´óµ½Đ¡µÄÅÅÁĐ˳Đ̣ÊÇ__________________¡£
![]() ·Ö±đỊ̈µÈ̀å»ư¡¢ÏàͬpHµÄHClÈÜ̉ººÍ
·Ö±đỊ̈µÈ̀å»ư¡¢ÏàͬpHµÄHClÈÜ̉ººÍ![]() ÈÜ̉ºÖĐ¼ÓÈë×ăÁ¿µÄZn·Û£¬·´Ó¦¸Ơ¿ªÊ¼Ê±²úÉú
ÈÜ̉ºÖĐ¼ÓÈë×ăÁ¿µÄZn·Û£¬·´Ó¦¸Ơ¿ªÊ¼Ê±²úÉú![]() µÄËÙÂÊ£º
µÄËÙÂÊ£º![]() ______
______![]() ̀î¡°
̀î¡°![]() ¡±¡¢¡°
¡±¡¢¡°![]() ¡±»̣¡°
¡±»̣¡°![]() ¡±ÏÂͬ
¡±ÏÂͬ![]() £¬·´Ó¦ÍêÈ«ºó£¬ËùµĂÇâÆøµÄÖÊÁ¿£º
£¬·´Ó¦ÍêÈ«ºó£¬ËùµĂÇâÆøµÄÖÊÁ¿£º![]() ÑÎËá_______
ÑÎËá_______![]() ´×Ëá¡£
´×Ëá¡£
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
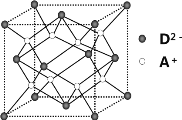
¡¾̀âÄ¿¡¿ÓĐA¡¢B¡¢C¡¢DËÄÖÖÔªËØ£¬ÆäÖĐAÔªËغÍBÔªËصÄÔ×Ó¶¼ÓĐ1¸öδ³É¶Ôµç×Ó£¬A£«±ÈB£ÉÙ̉»¸öµç×Ó²ă£¬BÔ×ÓµẲ»¸öµç×Ó̀îÈë3p¹́µÀºó£¬3p¹́µÀ̉ѳäÂú£»CÔ×ÓµÄp¹́µÀÖĐÓĐ3¸öδ³É¶Ôµç×Ó£¬ÆäÆø̀¬Ç⻯ÎïÔÚË®ÖеÄÈܽâ¶ÈÔÚͬ×åÔªËØËùĐγɵÄÇ⻯ÎïÖĐ×î´ó£»DµÄ×î¸ß»¯ºÏ¼ÛºÍ×îµÍ»¯ºÏ¼ÛµÄ´úÊưºÍΪ4£¬Æä×î¸ß¼ÛÑơ»¯ÎïÖĐº¬DµÄÖÊÁ¿·ÖÊưΪ 40£¥£¬Ç̉ÆäºËÄÚÖÊ×ÓÊưµÈÓÚÖĐ×ÓÊư¡£RÊÇÓÉA¡¢DÁ½ÔªËØĐγɵÄÀë×Ó»¯ºÏÎÆäÖĐA£«ÓëD2£Àë×ÓÊưÖ®±ÈΪ2¡Ă1¡£Çë»Ø´đÏÂÁĐÎỀ⣺
(1)B£µÄµç×ÓÅŲ¼Ê½Îª__________£¬ÔÚCB3·Ö×ÓÖĐCÔªËØÔ×ÓµÄÔ×Ó¹́µÀ·¢ÉúµÄÊÇ__________ÔÓ»¯¡£
(2)CµÄÇ⻯ÎïµÄ¿Ơ¼ä¹¹ĐÍΪ__________£¬ÆäÇ⻯ÎïÔÚͬ×åÔªËØËùĐγɵÄÇ⻯ÎïÖзеă×î¸ßµÄỘ̉ÊÇ__________¡£
(3)BÔªËصĵ縺ĐÔ__________DÔªËصĵ縺ĐÔ(̀î¡°£¾¡±¡¢¡°£¼¡±»̣¡°£½¡±)£¬ÓẲ»¸ö»¯Ñ§·½³̀ʽ˵Ă÷B¡¢DÁ½ÔªËØĐγɵĵ¥ÖʵÄÑơ»¯ĐÔÇ¿Èơ£º__________¡£
(4)ÈçͼËùʾÊÇRĐγɵľ§̀åµÄ¾§°û£¬É辧°ûµÄÀⳤΪa cm¡£ ÊÔ¼ÆËăR¾§̀åµÄĂܶÈΪ__________¡£(°¢·ü¼ÓµÂẪ³£ÊưÓĂNA±íʾ)
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
¡¾̀âÄ¿¡¿Ë®ĐÇ´óÆøÖĐº¬ÓĐ̉»ÖÖ±»³ÆΪẠ́»¯ôÊ(»¯Ñ§Ê½ÎªCOS)µÄÎïÖÊ¡£̉ÑÖªẠ́»¯ôÊÓëCO2µÄ½á¹¹ÏàËÆ£¬µ«ÄÜÔÚO2ÖĐÍêȫȼÉƠ£¬ÏÂÁĐÓĐ¹ØẠ́»¯ôʵÄ˵·¨ƠưÈ·µÄÊÇ£¨ £©
A.Ạ́»¯ôʵĵç×ÓʽΪ![]()
B.Ạ́»¯ôÊ·Ö×ÓÖĐÈư¸öÔ×ÓλÓÚͬ̉»Ö±ÏßÉÏ
C.Ạ́»¯ôÊµÄ·Đµă±È¶₫Ñơ»¯̀¼µÄµÍ
D.Ạ́»¯ôÊÔÚO2ÖĐÍêȫȼÉƠºóµÄ²úÎïÊÇCOºÍSO2
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
¡¾̀âÄ¿¡¿ÍÓë̉»¶¨Á¿Å¨ÏơËá·´Ó¦£¬µĂµ½ÏơËáÍÈÜ̉ººÍNO2¡¢N2O4¡¢NOµÄ»́ºÏÆø̀壬ƠâĐ©Æø̀åÓë11.2 L O2(±ê×¼×´¿ö)»́ºÏºóͨÈëË®ÖĐ£¬ËùÓĐÆø̀åÍêÈ«±»Ë®ÎüÊƠÉú³ÉÏơËá¡£ỘÏûºÄ͵ÄÖÊÁ¿Îª
A. 32 g B. 48 g C. 64 g D. 96 g
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
¡¾̀âÄ¿¡¿£¨1£©HCl¡¢NH4Cl¡¢CH3COOHºÍCH3COONaÊÇÖĐѧ³£¼ûµÄÎïÖÊ¡£
¢Ù³£ÎÂÏÂÔÚpH=7µÄCH3COOHºÍCH3COONaµÄ»́ºÏ̉ºÖĐc(Na+)_______c(CH3COO£)£῭î¡°£¾¡±¡¢¡°£¼¡±»̣¡°£½¡±£©£»
¢Ú³£ÎÂÏÂpH¾ùΪ6µÄHClºÍNH4ClÁ½ÖÖÈÜ̉ºÖĐ£¬ÉèÓÉË®µçÀë²úÉúµÄH+Ũ¶È·Ö±đΪamol/LºÍbmol/L£¬ỘaÓëbµÄ¹ØϵÊÇ_______£»
A£®a=b B£®a£½100b C£®b£½100a
£¨2£©T¡æ£¬ÔÚ̉»¸ö̀å»ưΪ2LµÄÈƯÆ÷ÖĐ£¬AÆø̀åÓëBÆø̀å·´Ó¦Éú³ÉCÆø̀壬·´Ó¦¹ư³̀ÖĐA¡¢B¡¢CÎïÖʵÄÁ¿±ä»¯ÈçͼËùʾ¡£
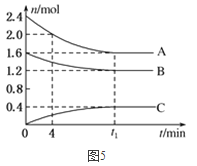
¢ÙT¡æʱ£¬¸Ă·´Ó¦µÄ»¯Ñ§·½³̀ʽΪ________£»
¢Ú0¡«4·ÖÖÓʱ£¬AµÄƽ¾ù·´Ó¦ËÙÂÊΪ_______molL£1min£1£»
¢Û´ïµ½Æ½ºâʱBµÄת»¯ÂÊΪ________£»
¢ÜT¡æʱ£¬¸Ă·´Ó¦µÄƽºâ³£ÊưΪ________¡£
£¨3£©½«TiO2ת»¯ÎªTiCl4Êǹ¤̉µ̉±Á¶½đÊôîѵÄÖ÷̉ª·´Ó¦Ö®̉»¡£
̉ÑÖª£ºTiO2(s)£«2Cl2(g)£½TiCl4(l)£«O2(g)¦¤H£½£«140.5kJ/mol
C(s£¬Ê¯Ä«)£«![]() O2(g)£½CO(g) ¦¤H£½£110.5kJ/mol
O2(g)£½CO(g) ¦¤H£½£110.5kJ/mol
Ộ·´Ó¦TiO2(s)£«2Cl2(g)£«2C(s£¬Ê¯Ä«)£½TiCl4(l)£«2CO(g)µÄ¦¤HÊÇ_______¡£
A£®£«80.5kJ/mol B£®£«30.0kJ/mol
C£®£30.0kJ/mol D£®£80.5kJ/mol
£¨4£©Cu(OH)2ÔÚË®ÈÜ̉ºÖĐ´æÔÚÈܽâƽºâ£ºCu(OH)2(s)![]() Cu2£«(aq)£«2OH£(aq)£¬Ksp£½c(Cu2£«)c2(OH£)£½2¡Á10£20¡£µ±ÈÜ̉ºÖи÷Àë×ÓŨ¶ÈĂƯµÄ³Ë»ư´óÓÚÈܶȻưʱ£¬Ộ²úÉú³Áµí£¬·´Ö®Î̃³Áµí¡£Ä³CuSO4ÈÜ̉ºÀïc(Cu2£«)£½0.02mol/L£¬Èç̉ªÉú³ÉCu(OH)2³Áµí£¬Ó¦µ÷ƠûÈÜ̉ºpH£¾________¡£
Cu2£«(aq)£«2OH£(aq)£¬Ksp£½c(Cu2£«)c2(OH£)£½2¡Á10£20¡£µ±ÈÜ̉ºÖи÷Àë×ÓŨ¶ÈĂƯµÄ³Ë»ư´óÓÚÈܶȻưʱ£¬Ộ²úÉú³Áµí£¬·´Ö®Î̃³Áµí¡£Ä³CuSO4ÈÜ̉ºÀïc(Cu2£«)£½0.02mol/L£¬Èç̉ªÉú³ÉCu(OH)2³Áµí£¬Ó¦µ÷ƠûÈÜ̉ºpH£¾________¡£
£¨5£©Ă£Ă£ºÚ̉¹ÖĐ£¬º½±êµÆΪº½º£Ô±Ö¸Ă÷ÁË·½Ị̈£®º½±êµÆµÄµçÔ´±ØĐë³¤Đ§¡¢Îȶ¨¡£Î̉¹ú¿Æ¼¼¹¤×÷ƠßÑĐÖƳö̉ÔÂÁºÏ½đ¡¢Pt£FeºÏ½đÍøΪµç¼«²ÄÁϵĺ£Ë®µç³Ø¡£
¢Ù¸Ăµç³ØÖĐ£¬º£Ë®Êǵç½âÖÊÈÜ̉º£¬¸º¼«²ÄÁÏ_______£῭î¡°ÂÁºÏ½đ¡±»̣¡°Pt£FeºÏ½đÍø¡±£©£»
¢Ú¸Ăµç³ØÖиº¼«µç¼«·´Ó¦Ê½Îª_______¡£
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
¡¾̀âÄ¿¡¿ÀûÓĂÈçͼËùʾװÖĂ£¬¿É̉ÔÄ£ÄầúµÄµç»¯Ñ§·À»¤¡£ÏÂÁĐ˵·¨´íÎóµÄÊÇ(¡¡¡¡)

A.ÈôXÊÇĐ¿°ô£¬½«KÓëMÁ¬½Ó£¬´Ë·½·¨ÊÇÎ₫ÉüÑô¼«µÄ̉ơ¼«±£»¤·¨£¬Ê¹̀ú²»̉×Êܸ¯Ê´
B.ÈôXÊÇ̀¼°ô£¬½«KÓëNÁ¬½Ó£¬¿É¼ơ»º̀úµÄ¸¯Ê´
C.ÈôXÊÇ̀¼°ô£¬½«KÓëMÁ¬½Ó£¬̀¼°ôµÄµç¼«·´Ó¦Ê½ÊÇ2H£«£«2e£=H2¡ü
D.ÈôXÊÇĐ¿°ô£¬½«KÓëNÁ¬½Ó£¬Đ¿°ôµÄµç¼«·´Ó¦Ê½ÊÇZn£2e£=Zn2£«
²é¿´´đ°¸ºÍ½âÎö>>
¹ú¼ÊÑ§Đ£ÓÅÑ¡ - Á·Ï°²áÁбí - ÊỒâÁбí
º₫±±Ê¡»¥ÁªÍøÎ¥·¨ºÍ²»Á¼ĐÅÏ¢¾Ù±¨Æ½̀¨ | ÍøÉÏÓĐº¦ĐÅÏ¢¾Ù±¨×¨Çø | µçĐÅƠ©Æ¾Ù±¨×¨Çø | ÉæÀúÊ·ĐéÎ̃Ö÷̉åÓĐº¦ĐÅÏ¢¾Ù±¨×¨Çø | ÉæÆóÇÖȨ¾Ù±¨×¨Çø
Î¥·¨ºÍ²»Á¼ĐÅÏ¢¾Ù±¨µç»°£º027-86699610 ¾Ù±¨ÓÊÏ䣺58377363@163.com