
ij��ѧС���Ա�����Ϊԭ����ȡ������������й����ʵķе����Է������������
I.�ϳɱ���������ֲ�Ʒ
����ƿ�м���12.2g�������20mL�״����ܶ�Լ0.79g/mL�� ����С�ļ���3mL Ũ���ᣬ���Ⱥ�Ͷ�뼸�����Ƭ��С�ļ���ʹ��Ӧ��ȫ���ñ���������ֲ�Ʒ��
��1���÷�Ӧ��Ũ��������� ������Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ ���״�������ԭ�� ��
��2���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ������ ��
��3���ס��ҡ�����λͬѧ�ֱ��������ͼ����ʵ������ȡ�����������װ�ã��г������ͼ�������������ȥ���������л�����ص㣬��ò��� װ�ã���ס������ҡ�������������
�ֲ�Ʒ�ľ���
��4������������ֲ�Ʒ���������������״������ᡢ�������ˮ�ȣ���������������ͼ���о��ƣ����������ͼ����ǡ���������������ƣ�����IΪ ������IIΪ ��
��5����������ͼ�м���Na2CO3��Һ�����Һ©���������ã�Ҫ�õ��л��㣬���������� ��
��6������������IJ���Ϊ ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�мס��ҡ����������ʣ�


�� �� ��
��1������������������ȷ����
a��1mol���л����ڼ��Ⱥʹ��������£�����ܺ�2molNaOH��Ӧ
b�����л�����ʹ����KMnO4��Һ��ɫ
c�����л���ķ���ʽΪC9H11Cl
d�����л�����һ�������£��ܷ�����ȥ��Ӧ��ȡ����Ӧ��������Ӧ�ͻ�ԭ��Ӧ
��2���ɼ�ת��Ϊ���辭���й��̣�����ȥ������Ӧ���ز����ͬ����
�� ��Ӧ��ķ�Ӧ������_________________________________________
�����з�ӦII�Ļ�ѧ����ʽ��
��3���ɼ׳����ϳɱ���·��֮һ���£�
д����Ӧ�۵Ļ�ѧ����ʽ__________________________________________________
��д�����Ľṹ��ʽ_________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
3��24g���ۺ�3��42g���ǻ�ϣ���һ��������ˮ�⣨������ȫˮ�⣩�����õ�ag�����Ǻ�bg���ǣ���a��b�ı�ֵ�Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С��������װ�ý����Ҵ���������ʵ�顣
��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ_________________��___________���ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵���Ҵ���������Ӧ��________��Ӧ��
��2����������ˮԡ�����ò���ͬ����������________���ҵ�������___________________��
��3����Ӧ����һ��ʱ����Թ�a�����ռ�����ͬ�����ʣ�������__________������ƿ���ռ������������Ҫ�ɷ���________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
��֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH
���й��л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/ �� | 34.7 | 78.5 | 118 | 77.1 |
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/�� | 34.7 | 78.5 | 118 | 77.1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ����С��̽������ˮ���������������������ʵ�顣
��1��ʵ����Ʒ��0.5 g���ۡ�4 mL 20%������Һ������������Һ��������Һ����ˮ��
���裺���Թ�1���Թ�2�������0.5 g���ۣ����Թ�1�����4 mL 20%������Һ�����Թ�2�����4 mLˮ��������3��4 min���ù�����Һ�к��Թ�1���������Һ����һ����Һ�嵹���Թ�3�����Թ�2��3�ﶼ�����ˮ���۲���û����ɫ���֡����Թ�1�����������Һ���Լ��Ⱥ۲��Թ��ڱ��������������֡�
������ѧ֪ʶԤ����ܵ�ʵ������
ʵ����������ۣ������±�����
| �Թ� | �����ˮ | ����������Һ | ���� |
| 1 | | | |
| 2 | | | |
| 3 | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������Ӧ�Ǽ���ȩ������Ҫ��Ӧ���̲ĶԸ�ʵ��IJ�������ֻ�Ǵ��Ե�������ijͬѧ���������о���
��1���ڸɾ��Թ��м���2ml2�� ��Ȼ�� �õ�������Һ����װ��5֧�Թܣ����Ϊ1#��2#��3#��4#��5# ��
��2�����εμ�2%��5%��10%��20%��40%����ȩ��Һ4�Σ�����������60��~70���ˮԡ�С�3���Ӻ��Թ�1#δ�γɴ�����������Թ�5# �������������кڰߣ� �Թ�4# ��������������һ�㣬�Թ�2#��3# �γɹ�����������
���о���Ŀ���ǣ� ��
��3����ȩ����������Ӧ�Ļ�ѧ����ʽΪ�� ��
��4����������:��ǿ���������£�����������Һ����������������֤�ͶԱ�ʵ�����¡�
| װ�� | ʵ����� | �Թ��е�ҩƷ | ���� |
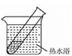 | ʵ��� | 2mL������Һ�����ν�ŨNaOH��Һ | �����ݲ���: һ��ʱ�����Һ���:�Թܱڸ������� |
| ʵ��� | 2mL������Һ�� ����Ũ��ˮ | �����ݲ���:һ��ʱ�����Һ�����Ա仯 |
 Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O
Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ij���������۾�����Ϊ����ͼ���ҵľۺ�������й�˵���������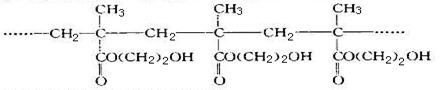
| A�����ɸþۺ���ķ�Ӧ���Ӿ۷�Ӧ |
| B���þۺ���ĵ����DZ������� |
| C��i�ۺ�������д��ڴ����ġ�OH�����Ծ��нϺõ���ˮ�� |
D���þۺ���ĽṹͲʽΪ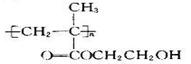 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���з�Ӧ���ڼӳɷ�Ӧ����
| A��2CH3CH2OH + 2Na ��2CH3CH2ONa + H2�� |
B�� |
C�� |
D�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com