
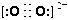 2NA��4��4 ��5��1s22s22p63s23p63d10 ��6��sp�ӻ� sp2�ӻ� 3
2NA��4��4 ��5��1s22s22p63s23p63d10 ��6��sp�ӻ� sp2�ӻ� 3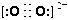 ����1��O22������2���м�����1 mol O22���У�����2NA���м���
����1��O22������2�������1 mol O22��������2NA�����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| Ԫ�� | Mn | Fe | |
| ������ /kJ��mol��1 | I1 | 717 | 759 |
| I2 | 1 509 | 1 561 | |
| I3 | 3 248 | 2 957 | |
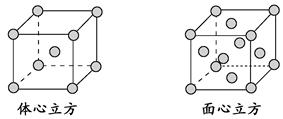
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
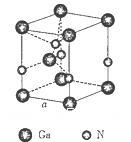
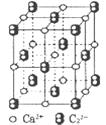
 ���г����㵪���ؾ����߳�a�ı���ʽ��a=_______cm��
���г����㵪���ؾ����߳�a�ı���ʽ��a=_______cm���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
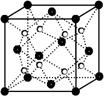

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A����ͬ���͵����Ӿ��壬������Խ���γɵľ���Խ�ȶ� |
| B�����Դ���ֻ��������Ҫ��һ�����Է��ӵĺϳ� |
| C���ý����ĵ����������ܺ����ؽ��ͽ�����ʴ��ԭ�� |
| D��H3O����NH4Cl��[Ag(NH3)2]���о�������λ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
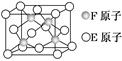
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com