

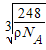 ��2�֣�
��2�֣� 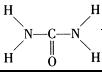 ��֪�����ط�����Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3��
��֪�����ط�����Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3�� +6��
+6��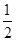 =4��V=
=4��V=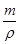 =
=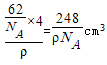 ����
����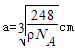 ��
�� 

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
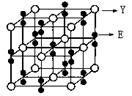
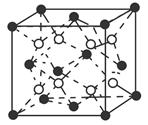
| Aԭ�Ӻ�����ӷ�ռ3����ͬ�ܼ�����ÿ���ܼ����Ų��ĵ�������ͬ |
| BԪ��ԭ�ӵĺ���p����������s����������1 |
| Cԭ��p����ϳɶԵ���������δ�ɶԵ�����������Aͬ���� |
| DԪ�ص����������������IJ�Ϊ4���Ҳ���AԪ����ͬһ���� |
| Eλ�����ڱ��е����� |
| FԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
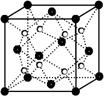
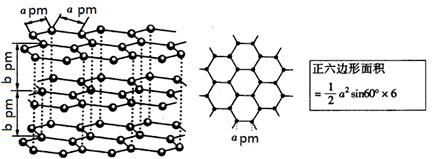
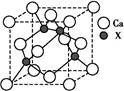
 Ϊ̼ԭ�ӣ�
Ϊ̼ԭ�ӣ� Ϊ��ԭ��)��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����________�����辧���߳�Ϊa cm���ܶ�Ϊb g��cm��3�����ӵ������ɱ�ʾΪ________(�ú�a��b��ʽ�ӱ�ʾ)��
Ϊ��ԭ��)��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����________�����辧���߳�Ϊa cm���ܶ�Ϊb g��cm��3�����ӵ������ɱ�ʾΪ________(�ú�a��b��ʽ�ӱ�ʾ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com