
����Ŀ���Ͼɹ��̽������к��н���Ag(�������������Բ���)���ӹ�������ȡ���� Ag �Ĺ����������¡���ش��������⡣

(1)�������������� 80 �� �����½��У�ʹ�õļ��ȷ�ʽΪ______________________��
(2)NaClO��Һ��Ag ��Ӧ�IJ���ΪAgCl��NaOH ��O2���÷�Ӧ�Ļ�ѧ����ʽΪ____________�����������HNO3���� NaClO����Ag���ӷ�Ӧ����ĽǶȷ�������ȱ����______________��
(3)�����ˢ���ϴ���������ʵ�����Ϊ_____________________________________��
(4)����10���İ�ˮ�ܽ�AgCl���壬 AgCl�� NH3H2O�� 1:2 ��Ӧ������ Cl����һ��������_____(�������ӵĻ�ѧʽ)����Һ��ʵ�ʷ�Ӧ�У���ʹ��ˮ����������������Ҳ��������AgCl���壬���ܵ�ԭ����__________________________________________��
(5)���������� 0.1 mol N2H4H2O�ɡ���ԭ���õ�_____ g Ag�ĵ��ʡ�
���𰸡�ˮԡ���� 4Ag+4NaClO+2H2O=4AgCl+4NaOH+O2�� ���ɵ��������Ⱦ���� ��©���м�������ˮ��������ȫ��û��ʹϴ��Һ��Ȼ���£��ظ�2~3�� ��Ag��NH3��2���� ��ˮ�ܽ�AgCl�ķ�Ӧ�ǿ��淴Ӧ�����ܽ��е��� 43.2
��������
(1)��ȷ�����¶ȣ���Ҫˮԡ���ȣ�
(2)���ݵ�ʧ�����غ���ƽNaClO��Һ��Ag ��Ӧ�ķ���ʽ��
(3)����ʵ������淶�ش�ϴ��������IJ���������
(4) ����ԭ���غ��ж�AgCl�� NH3H2O�� 1:2 ��Ӧ���ɵ������ӣ�
(5)���ݵ�ʧ�����غ��������Ag��������
(1)�������������� 80 �� �����½��У�ʹ��ˮԡ���ȣ�
(2)���ݵ�ʧ�����غ㣬NaClO��Һ��Ag ��Ӧ����AgCl��NaOH ��O2�ķ���ʽ��4Ag+4NaClO+2H2O=4AgCl+4NaOH+O2������������������������ԭ�����ǵ����������Ⱦ������
(3)�����ˢ���ϴ���������ʵ�����Ϊ��©���м�������ˮ��������ȫ��û��ʹϴ��Һ��Ȼ���£��ظ�2~3��
(4) AgCl�� NH3H2O�� 1:2 ��Ӧ����ʽ��AgCl+2NH3H2O![]() ��Ag��NH3��2��Cl���������ɵ��������ǣ�Ag��NH3��2���������ڿ��淴Ӧ���ܽ��е��ף����Լ�ʹ��ˮ����������������Ҳ��������AgCl���壻
��Ag��NH3��2��Cl���������ɵ��������ǣ�Ag��NH3��2���������ڿ��淴Ӧ���ܽ��е��ף����Լ�ʹ��ˮ����������������Ҳ��������AgCl���壻
(5) N2H4H2O���Ag��NH3��2��Cl ����������ԭ��Ӧ�����������ǵ�������ԭ������Ag��0.1 mol N2H4H2O��Ӧת��0.4mol���ӣ����ݵ�ʧ�����غ㣬�ɡ���ԭ���õ�0.4mol Ag������Ϊ0.4mol��108g/mol= 43.2g��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�������ö������̺�Ũ���ᷴӦ��ȡ�����Ļ�ѧ����ʽΪMnO2+4HCl��Ũ��![]() MnCl2+2H2O+Cl2������ش��������⣺
MnCl2+2H2O+Cl2������ش��������⣺
��1��������Ӧ�� ______ Ԫ�صĻ��ϼ۽��ͣ��� ______ ������������������ԭ������
��2��������0.1mol MnO2����ԭ�������ʵ����� ______ mol����Ӧ������ת�Ƶ��ӵ����ʵ���Ϊ ______ mol��
��3��������Ӧ��Ũ������ֳ��Ļ�ѧ������ ______ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��K3[Fe(C2O4)3]��3H2O�������������أ�Ϊ����ɫ���壬������ɹ����ͼ���ش��������⣺
��1��ɹ����ͼʱ����K3[Fe(C2O4)3]��3H2O���й������K3[Fe(CN)6]��ҺΪ��ɫ�������ⷴӦ�Ļ�ѧ����ʽΪ��2K3[Fe(C2O4)3]![]() 2FeC2O4+3K2C2O4+2CO2������ɫ��Ӧ�Ļ�ѧ����ʽΪ______________��
2FeC2O4+3K2C2O4+2CO2������ɫ��Ӧ�Ļ�ѧ����ʽΪ______________��
��2��ijС��Ϊ̽�������������ص��ȷֽ�������ͼ��ʾװ�ý���ʵ�顣
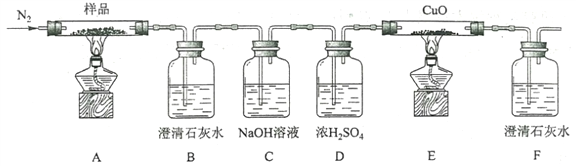
��ͨ�뵪����Ŀ����________________________________________��
��ʵ���й۲쵽װ��B��F�г���ʯ��ˮ������ǣ�װ��E�й����Ϊ��ɫ���ɴ��ж��ȷֽ������һ������___________��___________��
��Ϊ��ֹ������ֹͣʵ��ʱӦ���еIJ�����_____________________________��
����Ʒ��ȫ�ֽ��װ��A�еIJ����ﺬ��FeO��Fe2O3������Fe2O3���ڵķ����ǣ�________________��
��3���ⶨ�����������������ĺ�����
�ٳ���m g��Ʒ����ƿ�У��ܽ���ϡH2SO4�ữ����c mol��L-1 KMnO4��Һ�ζ����յ㡣�ζ��յ��������___________________________��
����������Һ�м������п������Ӧ��ȫ���ˡ�ϴ�ӣ�����Һ��ϴ��Һȫ���ռ�����ƿ�С���ϡH2SO4�ữ����c mol��L-1 KMnO4��Һ�ζ����յ㣬����KMnO4��ҺV mL���þ������������������ı���ʽΪ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���������
A. 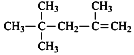 ��һ�ȴ�����4��
��һ�ȴ�����4��
B. ����������ϵĶ��ȴ�����6��
C. ![]() ��ͬ���칹�����������Һ���ͬ�����ŵ���3��
��ͬ���칹�����������Һ���ͬ�����ŵ���3��
D. 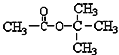 �ں˴Ź����������ܳ��������壬��������֮��Ϊ1��3
�ں˴Ź����������ܳ��������壬��������֮��Ϊ1��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʼ�������26 ����������˰����ӵ�����(NA��6.02214076��1023 mol�D1), ������2019 �� 5��20 ����ʽ��Ч������˵������ȷ����
A. �� 1 mol![]() �У����е�������Ϊ 6 NA
�У����е�������Ϊ 6 NA
B. ��7.1 g C12����ˮ�Ƴɱ�����ˮ��ת�Ƶĵ�����Ϊ 0.1 NA
C. ��״���£�11.2 L NO��11. 2 L O2��Ϻ�����ķ�������Ϊ 0.75 NA
D. ij�¶��£�1L pH= 3�Ĵ�����Һϡ�͵�10L ʱ����Һ�� H+����Ŀ����0.01 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������һ����Ҫ�Ļ�����Ʒ��ijѧϰС����Ʒ����Ʊ������ᡣ��Ӧԭ�����£�C6H5-CH3+2KMnO4![]() C6H5-COOK+KOH+2MnO2��+H2O��C6H5-COOK+HCl��C6H5-COOH+KCl����֪�ױ����۵�Ϊ��95�����е�Ϊ110.6�����ӷ����ܶ�Ϊ0.866g/cm3����������۵�Ϊ122.4������25����95�����ܽ�ȷֱ�Ϊ0.3g��6.9g��
C6H5-COOK+KOH+2MnO2��+H2O��C6H5-COOK+HCl��C6H5-COOH+KCl����֪�ױ����۵�Ϊ��95�����е�Ϊ110.6�����ӷ����ܶ�Ϊ0.866g/cm3����������۵�Ϊ122.4������25����95�����ܽ�ȷֱ�Ϊ0.3g��6.9g��
���Ʊ���Ʒ��30.0mL�ױ���25.0mL1mol/L���������Һ��80���·�Ӧ30min��װ����ͼ��ʾ��
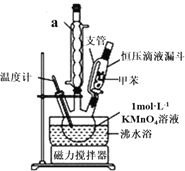
��1������a��������___��ͼ��֧�ܵ�������______��
��2��������þƾ���ֱ�Ӽ��ȣ��÷�ˮԡ���ȵ��ŵ���_____���ڱ�ʵ���У�������ƿ����ʵ��ݻ���___������ĸ����
A��50mL B��100mL C��200mL D��250mL
�������Ʒ����������������̷���ֲ�Ʒ������ͻ��ռױ���

��3����������������____�������ʵIJ��ᆳ��������һ���ᴿ����ɫҺ��A�����������������_____��
��4����������������_____������B�п��ܻ���________�������ӣ���������ӵķ�����_________���ᴿB���õķ�����_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ�������ع�ʽ������������⡣��ע����д��λ��
��1��3.01��1023��NH3���ӵ����ʵ���__��
��2��0.5molH2SO4���е���ԭ�ӵ����ʵ���__��
��3��2.3gNO2�����ʵ���__��
��4������£�33.6LN2�����ʵ���___��
��5��0.5mol /LK2SO4��Һ�У�c(K+)=__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2.32gNa2CO3��NaOH�Ĺ���������ȫ�ܽ���ˮ���Ƴ���Һ��Ȼ�������Һ����μ���1mol/L�����ᣬ�����������������CO2�����(��״��) ��ϵ��ͼ��ʾ������˵���д�����ǣ� ��
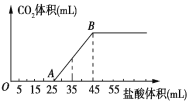
A.OA�η�����Ӧ�����ӷ���ʽΪ��H++OH-=H2O��CO32-+H+=HCO3-
B.A����Һ�е�����ΪNaCl��NaHCO3
C.�������NaOH������0.60g
D.������35mL����ʱ������CO2�����Ϊ224mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��A��B��C��D��E��ԭ���������������䵥�ʳ�������������̬���ֹ�̬��A��B�γɵ���̬�������ˮ��Һ��ʹ��ɫʯ����ֽ������A��Cͬ���壬Dԭ����������������Ӳ�����ȣ�E��ԭ�Ӱ뾶��ͬ������С������˵������ȷ���ǣ�
A. ԭ�Ӱ뾶��С��r(D) > r(E)
B. C����Ԫ���γɵĻ�������ܺ��зǼ��Լ�
C. E�γɵĵ��ʾ���Ư����
D. C��D������������Ӧ��ˮ����֮���ܷ�����Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com