
������ʵ��װ����ͼ��
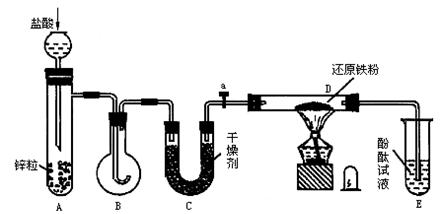
ʾ��ͼ��A�Ǽ���������������B�Ǵ�С���˵�Բ����ƿ��C��װ�и������U�ιܣ�a����ת������D��װ�л�ԭ���۵ķ�Ӧ�ܣ�E��װ�з�̪���Թܡ�
ʵ��ǰ�ȼ��ʵ��װ�õ������ԡ�ʵ�鿪ʼʱ���ȹرջ���a����ȡ����ƿB����A�м���һ�����ʵ�Ũ�ȵ����ᣬ��������������Ҫ�ġ��������������⣨2�������ڵ��ܵij��ڴ���ȼ������Ȼ����ͼ��ʾ������ƿB������ƿ������������ƿ�м���ȼ�գ��þƾ��Ƽ��ȷ�Ӧ��D�еĻ�ԭ���ۣ���B�������Ļ���Ϩ�����a������ͨ����Ӧ��D�����Թ�E�У�ʹ��̪��Һ�ʺ�ɫ����ش��������⡣
��1��ʵ��ǰ��μ��װ�õ������ԣ�</PGN0035B.TXT/PGN>____��
��2����ȼ����ǰ�������____���������иò����ķ�����____��
��3��д��B��D�зֱ�����Ӧ�Ļ�ѧ����ʽ��
B��_______________
D��________________
��4��C����ʢ�������������____���ø������������____��
(1)��A�з�������ˮ��ʹˮ��ս�û����©���¶ˣ�������a������ƿB�ײ��Լ��ȣ�����A©������ˮ����������E�е��ܿ��������ݳ�����ʾװ�ò�©����
(2)�����������ȣ�����ˮ��(�������ſ�����)�ռ�һС�Թ�H2����Ĵָ��ס�ƽ����棬�ƿ�Ĵָ����û�м���ı���������ʾH2���Ⱥϸ��Ե�ȼ���ռ���
![]()
(4)C�У���ʯ�ң���������������ˮ����������������
�����ۺϿ�����ʵ��װ�������Եļ�顢�������ȵļ��鼰NH3�ĺϳɵ�֪ʶ��
ѧ���ڻش�����ʱ�����׳��ֵĴ�����װ��������ʱϰ�����������ȷ�Ӧ���IJ���������������ʵ����ʹ�õĻ�ԭ�������Ƿ�Ӧ��Ӷ���ɴ�����жϡ����и�������E�е�������ʹ��̪��죬˵������������ˮӦ�Լ��ԣ�����ų���H2��O2�Ŀ����ԣ��ٸ���B��H2ȼ��ʱ���ĵ�O2��ʣ�������</PGN0087B.TXT/PGN>N2������ͬ���ɵ�H2�������������ڷ�Ӧ��D���ڻ�ԭ�����������������·�Ӧ����NH3���ݴ�д��D�з�Ӧ�Ļ�ѧ����ʽΪ��N2��3H2![]() 2NH3��
2NH3��


 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��| �������� | �����뾧�������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.6g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | �۵�/�� | �е�/�� | ��ѧ���� |
| S | 112.8 | 444.6 | �� |
| S2C12 | -77 | 137 | �� S2C12��ˮ����HCl��SO2��S��300��������ȫ�ֽ⣻ S2C12+C12 2SCl2 |
| SCl2 | -121 | 59.6���ֽ⣩ |

| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com