
| ||
| 2min |
| 0.6mol |
| 5mol |
| 0.4mol |
| 1.8mol+4.4mol+0.4mol |
| m |
| n |
| 1.8mol��28g/mol+4.4mol��2g/mol+0.4mol��17g/mol |
| 1.8mol+4.4mol+0.4mol |


 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��124g P4���е�P-P���ĸ���Ϊ4NA |
| B��12gʯī�к���C-C���ĸ���Ϊ1.5NA |
| C��12g���ʯ�к���C-C���ĸ���Ϊ2NA |
| D��60g SiO2�к�Si-O���ĸ���Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
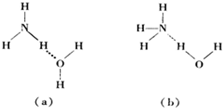 ��Ҫ�����
��Ҫ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
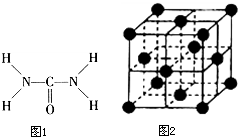 ��ͼ1����֪���صĽṹʽΪ�����ؿ��������л����ʣ���Ҫ�������������ᣬ�����غ���������ѧʽΪ[Fe��H2NCONH2��6]
��ͼ1����֪���صĽṹʽΪ�����ؿ��������л����ʣ���Ҫ�������������ᣬ�����غ���������ѧʽΪ[Fe��H2NCONH2��6]�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | X | Y | Z |
| ��ʼŨ�ȣ�mol/L�� | 0.1 | 0.2 | 0 |
| ƽ��Ũ�ȣ�mol/L�� | 0.05 | 0.05 | 0.1 |
| A����Ӧ�ﵽƽ��ʱ��X��ת����Ϊ50% |
| B����Ӧ�ﵽƽ��ʱ��Y��ת����Ϊ25% |
| C��������������ʱ������Y�����ʵ�����ƽ��������Ӧ�������ƶ���ƽ�ⳣ��K��� |
| D��������������ʱ�����¶����ߣ�ƽ�ⳣ��K����˵���÷�Ӧ����Ӧ��H��0 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com