
ʵ�飨1��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ�
�ٴ��ձ����粻��Ӳֽ�壬��õ��к�����ֵ ���ƫ����ƫС��������Ӱ�족����
��ʵ�����ܷ��û���ͭ˿��������滷�β�������� ����ܡ��������ܡ�����
��ijͬѧ��0.25mol/L��ϡ������������������ʵ�飬ʵ���������±�
i������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�� ��t2/�� | ƽ���¶Ȳ� (t2��t1)/�� | ||
| H2SO4��Һ | NaOH��Һ | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 |
|
| 2 | 27.0 | 27.4 | 27.2 | 32.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 |
ii��������Ϊ0.55 mol/L NaOH��Һ��0.25mol/L H2SO4��Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к��Ȧ�H��______(����С�����һλ)��
iii������ʵ��������ֵ��57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)____��
a��ʵ��װ�ñ��¡�����Ч���� b������ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ��ϡ�����С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
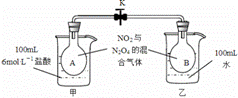 ��2����ͼ��ʾ����ƿA��B��װ����ͬŨ�ȵ�NO2��N2O4�Ļ�����壬�м���ֹˮ��K�н����ձ�����ʢ��100ml��6 mol /L��HCl��Һ���ձ�����ʢ��100mlˮ���������ˮ����ʼ�¶���ͬ�������ձ�����Һ�м���25gNaOH���壬ͬʱ���ձ����м���25gNH4NO3�������ʹ֮�ܽ�:
��2����ͼ��ʾ����ƿA��B��װ����ͬŨ�ȵ�NO2��N2O4�Ļ�����壬�м���ֹˮ��K�н����ձ�����ʢ��100ml��6 mol /L��HCl��Һ���ձ�����ʢ��100mlˮ���������ˮ����ʼ�¶���ͬ�������ձ�����Һ�м���25gNaOH���壬ͬʱ���ձ����м���25gNH4NO3�������ʹ֮�ܽ�:
��A��ƿ��������ɫ ����������ƽ�������� ����������С���������䡱����
��B��ƿ��������ɫ �������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�������õ�������250 mL0.2 mol��L-1 ��CuSO4��Һ��
(1)������Һʱ��һ����Է�Ϊ���¼������裺(������пո�)
A.���㡡B. ������C.�ܽ⡡��D. E. F. ���ݡ�G. ҡ�ȡ�װƿ��
(2)��ʵ������õ�����������ƽ��ҩ�ס����������ձ�����Ͳ����______
�� �����������ƣ�
(3) ��������Ҫ��ȡCuSO4��5H2O������Ϊ ��
(4) ����ȡ����ʱ���뱻��������(10g����������)�������Ƶ�CuSO4��Һ��Ũ�� (�ƫ�ߡ�����ƫ�͡�������Ӱ�족)��������ʱ���ӿ̶��ߣ������Ƶ�CuSO4��Һ��Ũ�� (�ƫ�ߡ�����ƫ�͡�������Ӱ�족)��
(5)��ȷ���ƺõ�CuSO4��Һ��ȡ��50mL ������50mL CuSO4��Һ�����ʵ���Ũ��Ϊ______________������Cu2+������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�о���Ա���Ƴ�һ���ˮ��أ�����Ϊ����DZͧ�Ĵ�����Դ���õ���Խ���﮺ְ�Ϊ�缫���ϣ���LiOHΪ����ʣ�ʹ��ʱ����ˮ���ɷŵ硣���ڸõ�ص�����˵������ȷ���ǣ� ��
A��ˮ���������������ܼ�
B���ŵ�ʱ����������������
C���ŵ�ʱOH���������ƶ� D���ܷ�ӦΪ��2Li��2H2O=== 2LiOH��H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵�����ʾ����ȷ���ǣ� ��
A���ɡ�C��ʯ����C���𣩣���H= +1.9 kJ/mol ����֪���ʯ��ʯī�ȶ�
B����101KPaʱ��1mol̼ȼ�����ų�������Ϊ̼��ȼ����
C����101kPaʱ��2gH2��ȫȼ������Һ̬ˮ���ų�285.8kJ����������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ��2H2(g)+O2(g) = 2H2O(l)����H= -571.6kJ/mol
D��HCl��NaOH��Ӧ���к��ȡ�H= -57.3kJ/mol����H2SO4��Ca(OH)2��Ӧ���к���Ϊ
��H= ��(2��57.3)kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�������£��������з�Ӧ��MgSO4(s) + CO(g)  MgO(s) + CO2(g) +SO2(g) ��H>0���÷�Ӧ�ں��ݵ��ܱ������дﵽƽ��������ı�ͼ�к�����x��ֵ�����´ﵽƽ���������y��x�仯���ƺ������ǣ� ��
MgO(s) + CO2(g) +SO2(g) ��H>0���÷�Ӧ�ں��ݵ��ܱ������дﵽƽ��������ı�ͼ�к�����x��ֵ�����´ﵽƽ���������y��x�仯���ƺ������ǣ� ��
| ѡ�� | x | y |
|
| A | �¶� | �����ڻ��������ܶ� | |
| B | CO�����ʵ��� | CO2��CO�����ʵ���֮�� | |
| C | SO2��Ũ�� | ƽ�ⳣ��K | |
| D | MgSO4����������������� | CO��ת���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.1026mol��L-1������ζ�25.00mLδ֪Ũ�ȵ�����������Һ���ζ����յ�ʱ���ζ����е�Һ������ͼ��ʾ����ȷ�Ķ���Ϊ(����)��
A. 22.30mL B. 22.35mL
C. 23.65mL D. 23.70 mL

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���0.10 mol��L��1 NaHCO3��Һ��˵����ȷ����(����)��
A�����ʵĵ��뷽��ʽΪNaHCO3��Na���� H���� CO32 ��
B��25 ��ʱ����ˮϡ�ͺ�n(H��)��n(OH��)�ij˻����
C������Ũ�ȹ�ϵ��c(Na��)��c(H��)��c(OH��)��c(HCO3�� )��c(CO32 ��)
D���¶����ߣ�c(HCO3�� )����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�ٱ������������춡�������ݼ��飬�������ʵķе㰴�ɵ͵��ߵ�˳�����е���(����)��
A���٢ۢڢܢݡ����£��ݢܢۢڢ١��ã��٢ڢۢܢݡ��ģ��ݢ٢ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���ǣ�����
��.CaCl2������״̬�¿ɵ��磬����ǿ�����
��.û�е��ʲμӵĻ��Ϸ�Ӧһ���Ƿ�������ԭ��Ӧ
��.Ԫ�ش������̬ʱһ������ǿ������
D����2KClO3+4HCl(Ũ)=2KCl+2ClO2+Cl2+2H2O�У��������������������ǻ�ԭ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com