
����Ŀ����̼�ֳ�����̼(��ѧʽΪCOS)����ũҩ��ҽҩ�������л��ϳɵ���Ҫԭ�ϡ�COS�ĺϳɷ���֮һ�������ܼ�����������CO����������Ӧ�Ƶã��÷����̼���Ч����������CO2��SO2�����ʡ�
��1��COS�ĵ���ʽΪ_________��
��2����֪CO2������ϳ��Ҵ��ķ�Ӧԭ����: 2CO2(g)+6H2(g)![]() C2H5OH+3H2O(g) ��H=-173.6kJ/mol
C2H5OH+3H2O(g) ��H=-173.6kJ/mol
��ͼ�Dz�ͬ��ʼͶ��ʱ��CO2��ƽ��ת�������¶ȱ仯�Ĺ�ϵ,ͼ��m=![]() ��Ϊ��ʼʱ��Ͷ�ϱȣ���m1��m2��m3�Ӵ�С��˳��Ϊ_______��������____________��
��Ϊ��ʼʱ��Ͷ�ϱȣ���m1��m2��m3�Ӵ�С��˳��Ϊ_______��������____________��

��3����Ȼ������������ȡ�Ļ���ԭ�����У�������COS��ĿǰCOSˮ�����ѳ�COS�ij�����������COS�ڴ�����������ˮ������Ӧ�������⣬���ɵ������������п��������ѳ���
��COSˮ��Ļ�ѧ����ʽΪ_________________��
�������£�ʵ��������(�ѳ�����)��Ӧ�����У�ÿ����4.05gZnO���ų�3.83kJ������������Ӧ���Ȼ�ѧ����ʽΪ_________________��
�����������绯ѧ�����������������ļ����õ��Ͽ췢չ���÷���������Fe3+��������������H2S��Ӧ�������ʣ���Ӧ�����Һ���õ��ķ�������������ʵ��ѭ�����á���ⷨʹFe3+�������������ӷ���ʽΪ___________���÷�Ӧ������������______��
�������£�HCl��CuCl2�Ļ����Һ�У�c(H+)=0.30mol/L��c(Cu2+)=0.10mol/L��������Һ��ͨ��H2S������(H2S�Ľ���Ũ��Ϊ0.10mol/L)��_____(������������������)���ֳ���,�ñ�Ҫ�ļ�����̺�����˵�����ɡ�
(��֪Ka1(H2S)=1.3��10-7��Ka2(H2S)=7.0��10-5��Ksp(CuS)=1.4��10-36)
���𰸡� ![]() m1>m2>m3 �¶���ͬʱ��Ͷ�ϱ�m��������H2������CO2ת�������� COS+H2O
m1>m2>m3 �¶���ͬʱ��Ͷ�ϱ�m��������H2������CO2ת�������� COS+H2O![]() CO2+H2S ZnO(s)+H2S(g)=ZnS(s)+H2O(l) ��H=-76.6kJ/mol Fe2++2H+
CO2+H2S ZnO(s)+H2S(g)=ZnS(s)+H2O(l) ��H=-76.6kJ/mol Fe2++2H+![]() 2Fe3++H2�� ̼���������ȶ��Ե缫���� ��
2Fe3++H2�� ̼���������ȶ��Ե缫���� ��
����������1��COS�ĵ���ʽΪ![]() ����2��m=
����2��m=![]() Խ��Խ�����ڶ�����̼��ת����������̼��ת����Խ��m1>m2>m3���������¶���ͬʱ��Ͷ�ϱ�m��������H2������CO2ת����������3����COSˮ�����ɶ�����̼�������ᣬ��Ӧ�Ļ�ѧ����ʽΪCOS+H2O
Խ��Խ�����ڶ�����̼��ת����������̼��ת����Խ��m1>m2>m3���������¶���ͬʱ��Ͷ�ϱ�m��������H2������CO2ת����������3����COSˮ�����ɶ�����̼�������ᣬ��Ӧ�Ļ�ѧ����ʽΪCOS+H2O![]() CO2+H2S���������£�ʵ��������(�ѳ�����)��Ӧ�����У�ÿ����4.05g��0.05molZnO���ų�3.83kJ��������������1molZnO��ų�76.6kJ������������Ӧ���Ȼ�ѧ����ʽΪZnO(s)+H2S(g)=ZnS(s)+H2O(l) ��H=-76.6kJ/mol������ⷨʹFe3+����������������������Fe2+ʧ���Ӳ���Fe3+��ͬʱ������������Ӧ�����ӷ���ʽΪFe2++2H+
CO2+H2S���������£�ʵ��������(�ѳ�����)��Ӧ�����У�ÿ����4.05g��0.05molZnO���ų�3.83kJ��������������1molZnO��ų�76.6kJ������������Ӧ���Ȼ�ѧ����ʽΪZnO(s)+H2S(g)=ZnS(s)+H2O(l) ��H=-76.6kJ/mol������ⷨʹFe3+����������������������Fe2+ʧ���Ӳ���Fe3+��ͬʱ������������Ӧ�����ӷ���ʽΪFe2++2H+![]() 2Fe3++H2�����÷�Ӧ������������̼���������ȶ��Ե缫���ϣ���c(H+)=0.30mol/L��c(Cu2+)=0.10mol/L��c(H2S)
2Fe3++H2�����÷�Ӧ������������̼���������ȶ��Ե缫���ϣ���c(H+)=0.30mol/L��c(Cu2+)=0.10mol/L��c(H2S)![]() 0.10mol/L��Ka1(H2S)=
0.10mol/L��Ka1(H2S)=![]() =
=![]() =1.3��10-7��
=1.3��10-7��![]() ��Ka2(H2S)=
��Ka2(H2S)=![]() =
=![]() =7.0��10-5��
=7.0��10-5��![]() ��Q sp(CuS)=
��Q sp(CuS)=![]()
![]() >Ksp(CuS)=1.4��10-36)�����ܳ��ֳ�����
>Ksp(CuS)=1.4��10-36)�����ܳ��ֳ�����


 ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ���ס������ص缫���϶���������̼������ش��������⣺

��1���������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�____________�����ҳ��е�____________����
���ҳ��������ĵ缫��Ӧʽ��___________________��
��2���������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ_________________ ��
�ڼ׳���̼���ϵ缫��Ӧʽ��_____________________��
�۽�ʪ���KI������ֽ�����ҳ�̼��������������ֽ��������Ӧ�����ӷ���ʽΪ_____________��
�����ҳ�ת��0.02 mol e����ֹͣʵ�飬������Һ�����200 mL������Һ����Ⱥ��pH��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ�������
A.�����£����ܱ�Ũ����ۻ�������������������Ũ����
B.��װʳƷ�ﳣ�й轺��ʯ�ҡ���ԭ��������С������������ͬ
C.Ũ��������ڸ���H2S��CO2
D.�Ͻ����������һ���������ֽ���������������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ijͬѧ�ú�������ķ���м��ȡ�Ȼ�����װ��(ʡ�Լг�װ�ã����������)������˵����ȷ����

A. װ��A�д��ڷ�Ӧ:2Fe3++Fe=3Fe2+
B. ʵ�鿪ʼʱ������a�������������װ��A��
C. װ��B���ռ����������ֱ�ӵ�ȼ
D. ��Ӧ����ձ���ͨ������SO2����Һ��ɫ�������ػ�ɫ��Ϊdz��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��ش��������⣺
��1����֪��������ȼ���Ȧ�H=-285.8 kJ/mol��������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ_____��
��2����ͼ��ʾ��101kPaʱ������������ȼ�������Ȼ�������������仯���˷�Ӧ���Ȼ�ѧ����ʽΪ________________��

��3����֪��1 molˮ�������Һ̬ˮ����44 kJ����ϱ��⣨1������2����Ϣ����֪��4HCl(g)��O2(g)![]() 2Cl2(g)��2H2O(g)����H��________ kJ/mol��
2Cl2(g)��2H2O(g)����H��________ kJ/mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������A(C9H12O)��һ�ַ��㴼�������ܱ�Cu��������������ҽҩ�����ϵĺϳɡ�A������ת����ϵ����ش��������⡣

��֪:
��
��R1COCH3+R2CHO![]() R1COCH=CHR2+H2O
R1COCH=CHR2+H2O
��1��A�Ľṹ��ʽΪ________��
��2��B��D��H��I�ķ�Ӧ���ͷֱ�Ϊ_____��______��
��3��F�еĺ�����������_______��(д����)
��4����JΪ����3����Ԫ����������H��J��Ӧ�Ļ�ѧ����ʽΪ__________��
��5�����й�������K��˵����ȷ������______��
A.K����ѧʽΪC14H12O
B.��ʹ���Ը��������Һ��ɫ
C.1mol��K��H2��ȫ�ӳ���Ҫ7mol��H2
D.K����������ԭ�ӿ�����ͬһƽ����
��6��H��ͬ���칹���У���������Ҫ���ͬ���칹����______�֡�
������FeCl3��Һ������ɫ��Ӧ��������NaHCO3��Һ��Ӧ��
���˴Ź���������ʾ��4�ֲ�ͬ��ѧ�������⣬�������Ϊ1:1:2:6��
��7�������ͪ(![]() )��һ����Ҫ��ҽҩ�м�������ο������ϳ���·�����һ�����嶡����(C(CH3)3Cl)�Ϳ�ȩ(
)��һ����Ҫ��ҽҩ�м�������ο������ϳ���·�����һ�����嶡����(C(CH3)3Cl)�Ϳ�ȩ(![]() )Ϊԭ���Ʊ������ͪ�ĺϳ���·(���Լ���ѡ)��__________
)Ϊԭ���Ʊ������ͪ�ĺϳ���·(���Լ���ѡ)��__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ձ�������鰷һֱ��Ϊ����ɭ��������ҩ������1998�걻���������в���A��Ⱦ�Լ��������ƣ�����鰷�ĺϳ�·����ͼ��ʾ������˵������ȷ����

A. ����齺�ķ���ʽ��C10H17N B. ����鰷��һ�����������
C. ��·���еķ�Ӧ������ȡ����Ӧ D. W��ͬ���칹����һ�����б���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ȿ����������̻��źŵ��ȡ���ҵ��������[��Ca(NO3)2��Ba(NO3)2������]���ᴿ�������¡�(��֪:�����ȡ����ᱵ������Ũ����)

��1��Ҫ�ӿ����������������ȡ�Ĵ�ʩ��______(дһ������)��
��2������1��������_______��ϴ�����õ�ϴ�Ӽ���______ .
��3������Һ2���й�����H2CrO4��N2H4��ԭΪCr3+��ͬʱ�ų�����Ⱦ�����壬д��������Ӧ�����ӷ���ʽ______�����������뻹ԭ��������ʵ���֮��Ϊ______ ��
��4����֪Cr(OH)3������ˮ����ԭ�����pH=8��Ŀ����_______ ��
��5��Ϊ�˲ⶨ������2����CrԪ�ص�������������������ʵ�顣(��֪:I2+2S2O32-=2I-+S4O62-)
![]()
�١�����2����CrԪ�ص���������Ϊ______(�ô���ʽ��ʾ)��
���������HI��Һ����̫�࣬�ⶨ�������_____(����ƫ��������ƫ����������Ӱ����)����ԭ����__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���F( )Ϊһ�ָ߷�����֬������C�ķ���ʽΪ:C10H10Obr2��F�ĺϳ�·������:
)Ϊһ�ָ߷�����֬������C�ķ���ʽΪ:C10H10Obr2��F�ĺϳ�·������:

��֪:��AΪ����ȩ��ͬϵ���������������Է�������Ϊ134��
��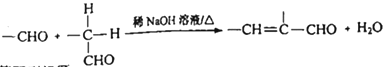
��ش���������:
��1��X�Ļ�ѧ������_____________________________________��
��2��E����F�ķ�Ӧ����Ϊ___________________________________��
��3��D�Ľṹ��ʽΪ________________________________��
��4����B����C�Ļ�ѧ����ʽΪ______________________________________��
��5�������廯����Y��D��ͬϵ�Y��ͬ���칹�����뱥��Na2CO3��Һ��Ӧ�ų����壬������ֻ��1���������˴Ź���������ʾ��5�ֲ�ͬ��ѧ�������⣬��ֵ�����Ϊ6:2:2:1:1��д�����ַ���Ҫ���Y�Ľṹ��ʽ____________��________________________��
��6��д���Լ�ȩ����ȩ���Ҷ���Ϊ��Ҫԭ�Ϻϳ����������۾��߷��Ӳ���-�ۼ���ϩ��������( )�ĺϳ�·��(���Լ���ѡ):__________________________��
)�ĺϳ�·��(���Լ���ѡ):__________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com