
����Ŀ�����ݻ�Ϊ![]() ���ܱ������г���
���ܱ������г���![]() �����
�����![]() ���壬��һ�������·������·�Ӧ��
���壬��һ�������·������·�Ӧ��![]() ����
����![]() ��ﵽƽ�⣬���C�����Ũ��Ϊ
��ﵽƽ�⣬���C�����Ũ��Ϊ![]() ������˵������ȷ����
������˵������ȷ����![]()
![]()
��������A��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ![]()
��������B��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ![]()
��ƽ��ʱ����A��B��ת�������
��ƽ��ʱ����B��Ũ��Ϊ![]()
�������������䣬���������ټ���![]() ���壬�ﵽ��ƽ��ʱ��C�������������
���壬�ﵽ��ƽ��ʱ��C�������������
A.�٢ڢ�B.�٢���C.�ۢ���D.�ڢۢ�
���𰸡�B
��������
��C��Ũ�ȱ仯Ϊ0.6mol/L������![]() ������v��C������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��A����
������v��C������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��A����
��C��Ũ�ȱ仯Ϊ0.6mol/L������![]() ������v��C������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��B����
������v��C������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��B����
��A��B��ѧ������֮��Ϊ2��1���μӷ�Ӧ��A��B�����ʵ���֮��Ϊ2��1��A��B����ʼ���ʵ���֮��Ϊ2��1��ƽ��ʱ����A��B��ת������ȣ�
�ܸ���C��Ũ�ȱ仯������B��Ũ�ȱ仯����ƽ��ʱ����B��Ũ�ȵ���B��ʼŨ�ȼ�ȥB��Ũ�ȱ仯����
��ԭƽ���뿪ʼ����3molC�ǵ�Ч�ģ���ԭƽ��״̬����1molC�����Ե�ЧΪ��ʼ����4molC���÷�Ӧ��Ӧǰ����������ʵ����仯���ں��º�������£�ѹǿ����ƽ��״̬��ͬ��
��C��Ũ�ȱ仯Ϊ0.6mol/L,����![]() ,����֮�ȵ��ڻ�ѧ������֮��,����
,����֮�ȵ��ڻ�ѧ������֮��,����![]() ���ʢ���ȷ��
���ʢ���ȷ��
���ɢ�֪,v(C)=0.3mol/(LS),����֮�ȵ��ڻ�ѧ������֮��,����![]() ���ʢڴ���
���ʢڴ���
��A. B��ѧ������֮��Ϊ2:1���μӷ�Ӧ��A. B�����ʵ���֮��Ϊ2:1��A. B����ʼ���ʵ���֮��Ϊ2:1��ƽ��ʱ����A��B��ת������ȣ��ʢ���ȷ��
�ܡ�c(C)=0.6mol/L,����![]() ��֪,
��֪, ![]() ������Bƽ��Ũ��Ϊ
������Bƽ��Ũ��Ϊ![]() ���ʢܴ���
���ʢܴ���
��ԭƽ���뿪ʼ����3molC�ǵ�Ч�ģ���ԭƽ��״̬����1molC�����Ե�ЧΪ��ʼ����4molC���÷�Ӧ��Ӧǰ����������ʵ����仯���ں��º�������£�ѹǿ����Ӱ��ƽ���ƶ�������3molC�����4molC�������ƽ��״̬��ͬ���������������ټ���1molC���壬�ﵽ��ƽ��ʱ��C������������䣬�ʢ���ȷ��
�٢ۢ���ȷ����ѡB��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1 mol SO2��1 mol O2ͨ�����������ܱ������У���һ�������·�����Ӧ2SO2(g)��O2(g)![]() 2SO3(g)���ﵽƽ��ʱSO3Ϊ0.3 mol����ʱ������0.5 mol O2��0.5 mol SO2����ͬ�¶����ٴδﵽ��ƽ��ʱSO3�����ʵ���Ϊ
2SO3(g)���ﵽƽ��ʱSO3Ϊ0.3 mol����ʱ������0.5 mol O2��0.5 mol SO2����ͬ�¶����ٴδﵽ��ƽ��ʱSO3�����ʵ���Ϊ
A. 0.3 mol B. 0.15 mol
C. ��0.15 mol D. ����0.15 mol����0.3 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С��Խ����ƽ����о�����֪C��D���ǵ��ʣ�F��ˮ��Һ��һ�ֳ�����ǿ�ᡣ

��1������Na�ڿ����з����㹻��ʱ�䣬���յ��������ǣ�_________________________��
��2����A��һ�ֳ����������ʣ���A��B��Һ�ܹ���Ӧ��������F��Һ��μ���E��Һ���ӱ�����������ʵ��������_________________________��
��3����A��һ�ֲ��ȶ����Σ�A��Һ��B��Һ��Ͻ�������ɫ��״������˲���Ϊ����ɫ������ɺ��ɫ��E����д���ù����з���������ԭ��Ӧ�Ļ�ѧ����ʽ��_________________________��
��4����A��һ����Һ��ֻ���ܺ���H+��NH![]() ��Mg2+��Fe3+��Al3+��CO
��Mg2+��Fe3+��Al3+��CO![]() ��SO
��SO![]() �е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ����������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е����Ӽ������ʵ���Ũ��֮��Ϊ_________��
�е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ����������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е����Ӽ������ʵ���Ũ��֮��Ϊ_________��
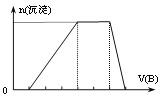
��5����NaHCO3��M�Ļ�������ܱ������г�ּ��Ⱥ��ų����壬���ⶨ�����ù���Ϊ�������NaHCO3��M��������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������β�������������ѳ�Ϊ����������ҵ��һ����Ҫ���⣬�����ԭ����ʵ��2NO��g��+2CO��g��![]() N2��g��+2CO2��g���ķ�Ӧ�����ڸ÷�Ӧ������˵����ȷ����
N2��g��+2CO2��g���ķ�Ӧ�����ڸ÷�Ӧ������˵����ȷ����
A.ֻҪʹ�ú����Ĵ��������壬�Ϳ���ȫ���NO��CO
B.ʹ�����ܺõĴ�����ʹ��Ӧ�ķ�Ӧ������
C.����÷�Ӧ���ܱ������н��У�һ��ʱ���ﵽƽ��ʱc��NO��=c��CO��
D.�÷�Ӧ�ﵽƽ��ʱv��NO����=v��CO����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪CH4(g)+2O2(g)��CO2(g)+2H2O(g) ��H== - Q1 ��
2H2(g)+O2(g) ��2H2O(g) ��H== - Q2��
H2O(g) ��H2O(l) ��H== - Q3
�����£�ȡ�����Ϊ4��1�ļ����H2�Ļ������112L����״���£�������ȫȼ�պ�ָ������£���ų�������Ϊ
A.4Q1+0.5Q2B.4Q1+Q2+10Q3C.4Q1+2Q2D.4Q1+0.5Q2+9Q3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.ijѧϰС����Na2O2��H2O��Ӧ��ʵ�飬����һЩ��Ȥ������
��ȡ����Na2O2��������֬�ް��÷���ʯ�����ϣ�Ȼ������֬���ϵμ�3-4��ˮ�������֬����ȼ�ա�
��ȡ����Na2O2���������Թ��У���ˮʹ���ַ�Ӧ�����ٲ�������Ϊֹ�����뼸�η�̪��Һ����Һ�ȱ�����ɫ���ش��й����⡣
��1��д��Na2O2�ĵ���ʽ_______________________��
��2����֪Na2O2�ɿ�����Ԫ����H2O2��Ӧ���Σ����һ��ˮ����ȫ���С�д�����һ��ˮ������ӷ���ʽΪ___________________________________��
��3���ɢ�ʵ���������ó����йؽ����ǣ�a.���������ɣ�b.___________________��
��4��Na2O2��H2O��Ӧ�����ӷ���ʽ____________________________________��
II.Ϊ̽��Na2O2��H2O��Ӧ�Ļ�������������ʦ��ָ�����������ͼ��ʾװ�á����Ӻ�װ�ã���K1��K2��ͨ��ע����ע����������ˮ����ַ�Ӧ�������������Na2S��Һ����ǣ�����KMnO4��Һ��ɫ���ֱ�ȡA��C����Һ���뼸�η�̪����ʼ����죬�Ժ�A����Һ�ܿ���ɫ��C����Һ������ɫ����ȡA��C����Һ�ֱ���������������̣���������־���Ӧ���ҡ������������ݣ��Ѵ����ǵ�ľ�������Թܣ�ľ����ȼ����Ӧ�����Һ�е��뼸�η�̪��Һ����Һ��첻��ɫ��
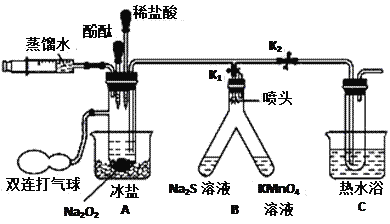
��5��A�б��κ�C����ˮ�����÷ֱ���__________________��___________________��
��6���û�ѧ����ʽ��ʾNa2S����ǵ�ԭ��___________________________________��
��7�������ӷ���ʽ��ʾKMnO4��Һ��ɫ��ԭ��MnO4�������������±���ԭ��Mn2+��__________________________________________��
��8��Na2O2��H2O��Ӧ�Ļ�����(�û�ѧ����ʽ��ʾ)��һ��_____________________���ڶ���_______________________________��
��9������Na2O2�еμ�������ϡ���ᣬҲ�ܲ���ͬ�������壬��д���÷�Ӧ�Ļ�ѧ����ʽ___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£��ں����ܱ������з������淴Ӧ:X2(g)+Y2(g)![]() 2Z(g)����֪X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.lmol/L��0.3mol/L��0.2mol/L������Ӧ�ﵽƽ��ʱ�������ʵ�Ũ�ȿ�����
2Z(g)����֪X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.lmol/L��0.3mol/L��0.2mol/L������Ӧ�ﵽƽ��ʱ�������ʵ�Ũ�ȿ�����
A. c(X2)=0.2mol/L B. c(Y2)=0.4mol/L
C. c(Z)=0.3mol/L D. c(X2)+c(Y2)+c(Z)=0.5mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��Ϊ̽�����ζ�ֲ��������Ӱ�������ȡ�����С����һ�¡���������Ľ�׳�����ÿ��磬�ֱ���ڵ�������A,B��ƿҺ��������������Aƿװ����ˮ. Bƿװ��������Һ������һ��ʱ���A��B��ƿֲ���������ͳ����ϵı仯���±���
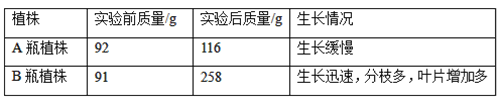
(1)Bƿֲ���������ӵ���Ҫԭ��������ֲ�����__________���ã��ϳ����л��
(2)A��B��ƿֲ�����յ�ˮ�ִ�ͨ��___________����ɢʧ�������У���ˣ�ֲ�����ֿ������ӿ�����ʪ�ȣ���������
(3)���ⶨÿ��Bƿ��Һ�к���Ԫ�ص�������1.12g����ô���������ƴ���Һʱ����ÿ��ˮ��Ӧ����___________gNH4NO3����������Һ�еĵ�Ԫ��ȫ����NH4NO3�ṩ���ܽ�����к�������仯��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������SO2��CO2��˵������ȷ���ǣ� ��
A. ����ֱ���νṹ
B. ����ԭ�Ӷ�����SP�ӻ����
C. SO2ΪV�νṹ��CO2Ϊֱ���νṹ
D. Sԭ�Ӻ�Cԭ���϶�û�й¶Ե���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com