
�ô����˫��ˮ��Ͽ���������Һ��ϴ�Ӽ�(2Na2CO3��3H2O2)��������ɱ������ȥ���۵������Ҳ�����ȾˮԴ��
(1)������������ϴ�Ӽ��н��������ӵIJ�����������_______________________________________��
(2)����ϴ�Ӽ��е�˫��ˮ���Խ���ˮ�е��軯��ת��Ϊ����ͬʱ����NH3��д����Ӧ�����ӷ���ʽ��___________________________________
(3)�������ϴ�Ӽ���ˮ�к��������ӣ�����������ϴ�Ӽ���ȥ��������������ȫʧȥɱ�����á��Է������е�ԭ��(�����ӷ���ʽ�ͼ�Ҫ���ֱ���)��__________________________________________________________________
(4)ij��ѧѧϰС��Ϊ����̽�������Ӷ���������ϴ�Ӽ��IJ���Ӱ�죬ȡ��ϴ�Ӽ�100 mL������25 gFeCl3���壬����������ɫ��ζ���壬������ƿ�ռ����塣��ѡ�������Լ���ʵ����Ʒ�������ɷֵ�̽�����̣�0.10 mol��L��1 NaOH��Һ��8.0 mol��L��1 NaOH��Һ������ʯ��ˮ��0.01 mol��L��1 KMnO4��Һ��BaCl2ϡ��Һ��Ʒ����Һ������ˮ��Сľ�����ƾ��ơ����ϴ��ƿ��
��������裺�Ը�����ɷ�����������衣
����1��������O2��
����2��������________________��
����3��������CO2��
����Ʒ��������ʵ�鷽��֤����ļ��裬���±������ʵ�鲽�衢Ԥ����������ۡ�
| ʵ�鲽�� | Ԥ����������� |
| ����������ͨ��ʢ��________��________��ϴ��ƿ�У�________ | __________________ |
��(1)�ýྻ�IJ�˿պȡϴ�Ӽ��ھƾ��ƻ��������գ�����ʻ�ɫ(��������)
(2)H2O2��CN����H2O=HCO3����NH3
(3)2H2O2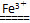 2H2O��O2���������ӻ����H2O2�ֽ⣬ʹϴ�Ӽ�ʧȥɱ�����ã�2Fe3����3CO32����3H2O=2Fe(OH)3����3CO2����Fe3����CO32��ˮ����ٽ���ʹϴ�Ӽ�ʧȥȥ������
2H2O��O2���������ӻ����H2O2�ֽ⣬ʹϴ�Ӽ�ʧȥɱ�����ã�2Fe3����3CO32����3H2O=2Fe(OH)3����3CO2����Fe3����CO32��ˮ����ٽ���ʹϴ�Ӽ�ʧȥȥ������
(4)��CO2��O2
��ʵ�鲽�� Ԥ����������� ����ʯ��ˮ
8.0 mol��L��1 NaOH��Һ�����������ǵ�Сľ���������һ��ϴ��ƿ�ij��ڴ�������ʯ��ˮ������ǣ�ľ����ȼ�������1������������ʯ��ˮ����ǣ�ľ����ȼ�������2������������ʯ��ˮ����ǣ�ľ������ȼ�������3����
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ�Ǿ����Դ���⣬��ҵ�ϴӺ�ˮ����ȡʳ�κ���Ĺ������£�
��1�������õ���-KI��ֽ�������I���Ƿ��������ɣ������������___________________��
��2������������Ӧ�����ӷ���ʽΪ_____________��������̼������Һ����������еĶ�������ˮ��Һ������������Ļ��ϼ۷ֱ�Ϊ+5��-1�ۣ��������������ϡ���������������������з�����Ӧ�����ӷ���ʽΪ______��
��3���屽��һ�ֻ���ԭ�ϣ���������ͱ���Ӧ�ϳɡ�ʵ���Һϳ��屽��װ��ʾ��ͼ���£�

�±�Ϊ��������屽��������ݣ�
| | �� | �� | �屽 |
| �ܶ�/g��cm-3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ǹ�ҵ���Ʊ�Na2S2O3�ķ���֮һ����Ӧԭ��Ϊ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2 ���÷�Ӧ��H>0��
ij�о�С����ʵ��������Ʊ�Na2S2O3��5H2O�������¡�
��1������װ����ͼ��ʾ��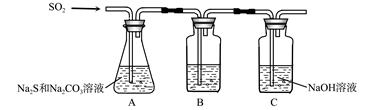
��װ��B�������Ǽ���װ��A��SO2������Ч�ʣ�B���Լ��� ������SO2����Ч�ʵ͵�ʵ��������B����Һ ��
��Ϊ��ʹSO2������������ȫ���ڲ��ı�A����ҺŨ�ȡ�����������£����˼�ʱ���跴Ӧ���⣬���ɲ�ȡ�ĺ�����ʩ�� �� ����д��������
��2�����豾ʵ�����õ�Na2CO3������NaCl��NaOH�����ʵ�鷽�����м��顣������ʱCaCO3������Һ��pH=10.2��
��ѡ�Լ���������ϡ���ᡢAgNO3��Һ��CaCl2��Һ��Ca��NO3��2��Һ����̪��Һ������ˮ��pH�ơ��ձ����Թܡ��ι�
| ��� | ʵ����� | Ԥ������ | ���� |
| �� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ⣬___________________�� | _______________ | ��Ʒ��NaCl |
| �� | ��ȡ������Ʒ���ձ��У�������������ˮ����ֽ����ܽ⣬___________________�� | _______________ | ��Ʒ��NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
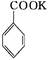
������㷺Ӧ������ҩ�ͻ�����ҵ��ijͬѧ�����üױ���������Ӧ�Ʊ������ᡣ��Ӧԭ��: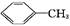 +2KMnO4
+2KMnO4
 +KOH+
+KOH+
2MnO2��+H2O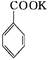 +HCl
+HCl
 +KCl
+KCl
ʵ�鷽��:һ�����ļױ���KMnO4��Һ��100 �淴Ӧһ��ʱ���ֹͣ��Ӧ,���������̷����������ͻ���δ��Ӧ�ļױ���
��֪:�����������122,�۵�122.4 ��,��25 ���95 ��ʱ�ܽ�ȷֱ�Ϊ0.3 g��6.9 g;���������л���һ�㶼�й̶��۵㡣
(1)��������������,����������������
(2)��ɫҺ��A����������,���Լ���A���Լ�����������,�������� ��
(3)�ⶨ��ɫ����B���۵�,��������115 �濪ʼ�ۻ�,�ﵽ130 ��ʱ�����������ۡ���ͬѧ�Ʋ��ɫ����B�DZ�������KCl�Ļ����,��������·��������ᴿ�ͼ���,ʵ���������Ʋ���ȷ������ɱ������ݡ�
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ɫ����B����ˮ��,�����ܽ�,������ | �õ���ɫ�������ɫ��Һ | |
| �� | ȡ������Һ���Թ���,�������� | ���ɰ�ɫ���� | ��Һ��Cl- |
| �� | �����ɫ����,������ | ������ | ��ɫ���� �DZ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ҷ���(H2C2O4)�׳Ʋ��ᣬ��һ����Ҫ�Ļ���ԭ�ϡ��������ϣ��˽�����й���Ϣ��
���Ҷ���������ˮ��������100�濪ʼ������125��ʱѸ��������157��ʱ������������ʼ�ֽ⡣�Ҷ������ȷֽ�����ˮ��������̼��һ�ֳ����Ļ�ԭ�����塣
���Ҷ���ĸ��Ρ����Ҷ����Ϊ������ˮ�İ�ɫ���塣
(1)��д���Ҷ������ȷֽ�Ļ�ѧ����ʽ______________________��
(2)��ѧ��ȤС���ͬѧ��ʵ��֤���Ҷ��ᾧ�����ȷֽ����ɵ�����ɷ֡�����������ͼ�ṩ��װ�ã���ѡ�Լ������������ʵ�鷽������A��B��C��C��C��D��E˳�������������װ�ã������Ҷ��ᾧ�����ȷֽ����ɵ�����ɷ֡�
���㰴����װ�ô������ҵ�˳����д�±��еĿո�
| �������� | �������������� | װ������ |
| B | | |
| C | | |
| C | ��������Ũ��Һ | |
| C | | |
| D | | |
| E | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
TiO2�����Ʊ��������ѻ������ԭ�ϣ�����һ����������İ�ɫ���ϡ�
(1)ʵ�������÷�ӦTiO2(s)��CCl4(g) TiCl4(g)��CO2(g)������ˮ�����������Ʊ�TiCl4��ʵ��װ��ʾ��ͼ���£�
TiCl4(g)��CO2(g)������ˮ�����������Ʊ�TiCl4��ʵ��װ��ʾ��ͼ���£�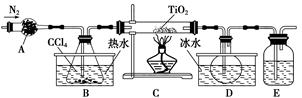
�й������������±���
| ���� | �۵�/�� | �е�/�� | ���� |
| CCl4 | ��23 | 76 | ��TiCl4���� |
| TiCl4 | ��25 | 136 | ����ʪ������������ |
 H2TiO3(s)��H2SO4(aq)
H2TiO3(s)��H2SO4(aq)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�뵼�������г���Ҫ���Ʋ���,�Ա�֤���Ƶ�����,���Ȼ���(PCl3)��һ����Ҫ�IJ��Ӽ���ʵ����Ҫ�û���(����)������Cl2ģ�ҵ������ȡPCl3,װ������ͼ��ʾ:(���ּг�װ����ȥ)
��֪����������Cl2��Ӧ����PCl3,�����Cl2��Ӧ����PCl5��PCl3��ˮ��ǿ��ˮ������H3PO3��HCl,��O2������POCl3,POCl3����PCl3��PCl3��POCl3���۷е���±�:
| ���� | �۵�/�� | �е�/�� |
| PCl3 | -112 | 75.5 |
| POCl3 | 2 | 105.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����ɷ��е�ļ���ʵ�鷽����ơ�
(1)�ɺ������պ�,�������ҷ���С�ձ��м�����������ˮ,���衢�����ȴ�����ˡ�����Һ�ֳ��ķݷ�����֧�Թ���,����Ϊ1��2��3��4�š�
(2)��1���Թ��е���6��ϡ�����,�ټ���Լ3 mL H2O2��Һ,����1%����Һ1~2��,�۲쵽��Һ�����ɺ�ɫ�����ɫ,���ӷ���ʽΪ��������������������������
(3)��2���Թ��м���2 mL���Ƶı�����ˮ,����Һ,�۲�����,���ӷ�Ӧ����ʽΪ����������������������������2 min��Ѽ�����ˮ����Һ�ֳ����ݡ����м����ٵ���1%����Һ1~2��,�۲�����Ϊ������������������,����Һ�м���2 mL CCl4,����ȡ,����2 min��۲�����Ϊ������������������������
(4)��3���Թ��м���ʳ�õ���3 g,��ʹ֮����ܽ�����6��ϡ���ᡣ�ڵ���1%����Һ1~2��,�۲쵽��Һ��������ɫ�����ɫ,��ص����ӷ���ʽΪ����������������������
(5)��4���Թ��м�����������Һ,��,�ټ���ϡ������Һ��ԭ�����÷�Ӧ���ɻ�ɫ��������������ӡ�ͨ��ʵ�鷢�����ɰ�ɫ�������ô˷��������Ԫ��ʧ�ܡ����´˲�ʧ�ܵĿ���ԭ��������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����к��зḻ�ĵ⡣Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
����д���пհף�
��1����������պ���ʱ������Ҫ���żܡ��������⣬����Ҫ�õ���ʵ��������
A���ձ� B�������� C������ D���ƾ��� E��Բ����ƿ
��2����д��������з�Ӧ�����ӷ���ʽ��
��3��������ǴӺ���ı���Һ�з�������ʵ�ͻ��ձ������辭������ָ������������ʵ��װ���еĴ���֮����������������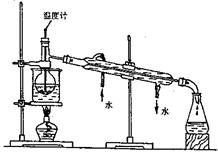
��
��
��4��������У�ijѧ��ѡ���ñ�����ȡ�⣬��������ɷֽ�Ϊ���¼���:
(A)��ʢ����Һ�ķ�Һ©����������̨����Ȧ�У�
(B)��50������ˮ��15�����������Һ©����,���Ǻò�������
(C)�����Һ©���������ϿڵIJ������Ƿ�©Һ��
(D)��ת©��������,����ʱ������������,���رջ������ѷ�Һ©��������
(E)��������,���ձ�������Һ��
(F)����Һ©���Ͽڵ����ϲ���Һ��
(G)��©���ϿڵIJ�������ʹ���ϵİ��ۻ�С��©�����ϵ�С�ף�
(H)���ã��ֲ㡣�ʹ�ʵ�飬����������:
����ȷ���������˳���� �� �� ��A��G�� ��E��F
������(G)���������Ŀ����
����ѡ�ñ��ӵ�ˮ����ȡ���ԭ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com