
����Ŀ��ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�
I.��1���ñ���������Һ�ζ����������������Һʱ,���ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��_________��ֱ�������һ���������Һ�ɻ�ɫ��Ϊ��ɫ��������ζ��յ㡣
��2�����в����п���ʹ��������������Һ��Ũ����ֵƫ�͵���_______________
A ��ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ
B �ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и���
C ��ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D ��ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
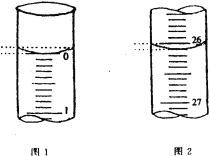
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ_________mL���յ����Ϊ_____________mL������������Һ�����Ϊ______________mL��
��4��ijѧ����������ʵ��ֱ��¼�й��������±���
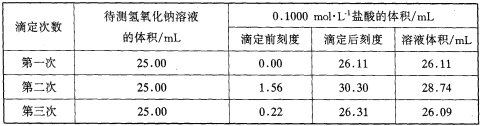
��ѡ�����к�����������ʽ���������������Һ�����ʵ���Ũ�ȣ�����4λС������c��NaOH��______
II.��H+Ũ����ͬ�������ȵ������a������b������ c�����ᣬͬʱ����������п����ʼ��Ӧʱ����_____________��Ӧ��ȫ������H2������ ___________����<��=��> ��ʾ��
���𰸡���ƿ����ɫ�仯 D 0.00mL 26.10mL 26.10mL 0.1044mol/L a=b=c a=b<c
��������
I.��1������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯��
��2��A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=![]() ����֪c������ƫ��ѡ��A����
����֪c������ƫ��ѡ��A����
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=![]() ����֪c���������䣬ѡ��B����
����֪c���������䣬ѡ��B����
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����c�����⣩=![]() ����֪c������ƫ��ѡ��C����
����֪c������ƫ��ѡ��C����
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=![]() ����֪c������ƫС��ѡ��D��ȷ��
����֪c������ƫС��ѡ��D��ȷ��
��ѡD��
��3����ʼ����Ϊ0.00mL���յ����Ϊ26.10mL��������Һ�����Ϊ26.10mL��
�������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ=26.10mL��
HCl+NaOH=NaCl+H2O
0.0261L��0.1000mol/L 0.025L��c��NaOH��
��c��NaOH��=![]() =0.1044mol/L��
=0.1044mol/L��
II.H+Ũ�ȴ�СӰ�����������ķ�Ӧ���ʣ�H+Ũ����ͬ����ʼʱ�ķ�Ӧ������ͬ����a=b=c��
����Ϊ���ᣬ������ȫ���룬��H+Ũ����ͬʱ�����Ũ�������������ᶼΪǿ�ᣬH+Ũ����ͬ����Һ�����ͬ����H+���ʵ�����ͬ������ȫ��Ӧ������H2������ɴ�С��c��a=b��


 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���25 mL0.12 mol/L AgNO3��Һ����μ���Ũ��Ϊ2%�İ�ˮ���ȳ��ֳ����������μӳ����ܽ⡣�ù����м��백ˮ�����V����Һ��lg �Ĺ�ϵ��ͼ��ʾ����֪e��ʱ��ҺѸ���ɻ��DZ�ó��壬�Ҵ�ʱ��Һ��c(Ag+)��c(NH3)��ԼΪ2��10-3 mol/L��������������ȷ���ǣ� ��
�Ĺ�ϵ��ͼ��ʾ����֪e��ʱ��ҺѸ���ɻ��DZ�ó��壬�Ҵ�ʱ��Һ��c(Ag+)��c(NH3)��ԼΪ2��10-3 mol/L��������������ȷ���ǣ� ��

A.a����Һ�����Ե�ԭ����AgNO3ˮ��
B.b����Һ�У�c(Ag+)+c[Ag(NH3)2+]��c(NO3-)
C.��e���֪����ӦAg++2NH3![]() [Ag(NH3)2]+ƽ�ⳣ����������Ϊ105
[Ag(NH3)2]+ƽ�ⳣ����������Ϊ105
D.c��d�μ���İ�ˮ��Ҫ���ڳ��������ɺ��ܽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.1 mol��L��1 CH3COOH��Һ�д������µ���ƽ�⣺ CH3COOH ![]() CH3COO����H+�����ڸ�ƽ�⣬����������ȷ���ǣ� ��
CH3COO����H+�����ڸ�ƽ�⣬����������ȷ���ǣ� ��
A. ����ˮʱ��ƽ�����淴Ӧ�����ƶ�
B. ��������NaOH���壬ƽ��������Ӧ�����ƶ�
C. ��������0.1 mol��L��1 HCl��Һ����Һ��c(H+)��С
D. ��������CH3COONa���壬ƽ��������Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ������ܴﵽʵ��Ŀ�ĵ���
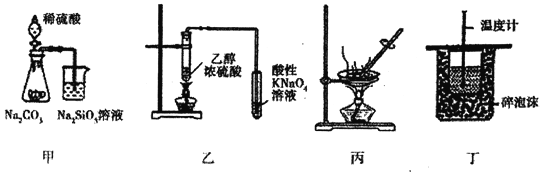
A. ͼ��װ�D��֤���ǽ�����ǿ����S>C>Si
B. ͼ��װ�ÿ����ڼ�������ϩ����
C. ͼ��װ�ÿ�ͨ������AlCl3������Һ�Ʊ�AlCl3����
D. ͼ��װ�ÿ������ⶨ�к���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������������������ǵ�����������ʡ�
(1)�γ������ԭ��֮һ�ɼ�ʾ���£�
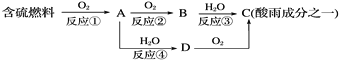
�ش��������⣺
�������pH________(���������������=��)5.6��
��D���ʵĻ�ѧʽΪ____________��
�۷�Ӧ�ڵĻ�ѧ����ʽΪ_________________________________________��
(2)��һ�������°��������������������ת��Ϊ����Ⱦ�����ʡ�д�������Ͷ���������һ�������·�Ӧ�Ļ�ѧ����ʽ��__________________����Ӧ����������____________����ԭ����_______________��
(3)������������Һ�������շ����еĵ��������Ӧ�Ļ�ѧ����ʽ���£�
NO2+NO+2NaOH===2NaNO2+H2O��2NO2+2NaOH===NaNO2+NaNO3+H2O
����VLijNaOH��Һ����ȫ����n molNO2��m molNO��ɵĴ�����Ⱦ�
�������ռ���Һ�����ʵ���Ũ������Ϊ________ mol��L1��
����������Һ��c(NO3��)��c(NO2��)=1��9����ԭ���������NO2��NO�����ʵ���֮��n��m=______��
���ú�n��m�Ĵ���ʽ��ʾ������Һ��NO3����NO2��Ũ�ȵı�ֵc(NO3��)��c(NO2��)=________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʢ��14 mL�ɶ���������������ɵĻ���������Ͳ������ˮ���У���ַ�Ӧ��ʣ��4 mL����(��ͬ״���²ⶨ)��ԭ��������ж�������������������ֱ��Ƕ��٣�____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����ʵ��Ʊ�װ�á��Ʊ�ʱʹ�õ�ԭ�����ռ���������ȷ���ǣ� ��
��� | ���� | �Ʊ�װ�� | �Ʊ�ʱʹ�õ�ԭ�� | �ռ����� | ��� | ���� | �Ʊ�װ�� | �Ʊ�ʱʹ�õ�ԭ�� | �ռ����� |
A |
|
|
| ��ˮ�� | B | NO |
| Ũ����ͽ���ͭ | �ſ����� |
C |
|
| Ũ��ˮ���������ƹ��� | �ſ����� | D |
|
| �Ҵ��� | ��ˮ�� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(��������ˮ������Ӧ)��һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ѧ�о�С��ͬѧ������������ȡ���������ⶨ���ò�����AlN������������
��֪��AlN��NaOH��3H2O��Na[Al(OH)4]��NH3����
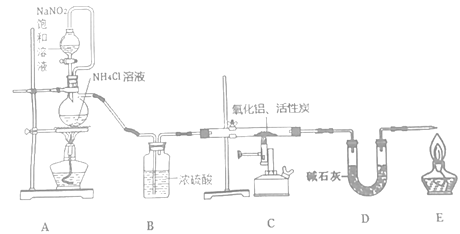
�ش��������⣺
��1�����װ�������ԣ�����ҩƷ����ʼʵ�顣���ȵ�ȼ___(��A������C����E��)���ľƾ��ƻ�ƾ���ơ�
��2��װ��A�з�����Ӧ�����ӷ���ʽΪ___��װ��C����Ҫ��Ӧ�Ļ�ѧ����ʽΪ___���Ƶõ�AlN�п��ܺ���������������̿����ܺ���___��
��3��ʵ���з��ֵ����IJ������ʹ��죬����Ӱ��β���Ĵ�����ʵ����Ӧ��ȡ�Ĵ�ʩ��___(д��һ�ִ�ʩ����)��
��4����ȡ5.0gװ��C�����ò������NaOH��Һ��������ɰ��������Ϊ1.68 L(��״��)�������ò�����AlN����������Ϊ___��
��5��Ҳ���������뵪����1000��ʱ��Ӧ��ȡAlN������������������NH4Cl���岢��ֻ�ϣ�������AlN���Ʊ�������Ҫԭ����___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ԭ���ԭ����ʵ��ʱ��������ʵ�鲽��:
���õ��߽����������Ƶ����˷ֱ��봿����пƬ��ͭƬ������(��ͼ1);
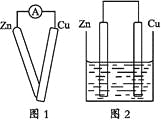
����һ�鴿����пƬ����ʢ��ϡ������ձ���;
����һ�鴿����ͭƬ����ʢ��ϡ������ձ���;
���õ��߰�пƬ��ͭƬ������������ƽ�еز���ʢ��ϡ������ձ���(��ͼ2)��
�ش���������:
(1)ʵ�鲽������Ӧ�۲쵽��������_______________________��
(2)ʵ�鲽������Ӧ�۲쵽��������_______________________��
(3)ʵ�鲽������Ӧ�۲쵽��������_______________________��
(4)ʵ�鲽������Ӧ�۲쵽��������_______________________��
(5)ͨ��ʵ�鲽������ͬѧͷ��������һ������(�����)���ò�����_______��
(6)Ϊ��֤ʵ�ò��룬��ͬѧ������˵�����ʵ�飬���Ҫ����������ʵ���װ��ʾ��ͼ��______
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com