
�������ƣ�NaClO2��������ˮ��������ɰ�ǡ���֬��Ư����ɱ�����������ù������ⷨ�����������ƵĹ�������ͼ��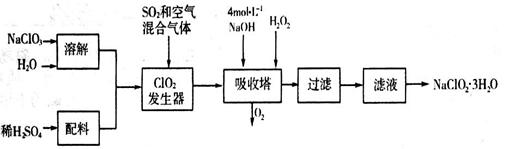
��֪��NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
��1���������ڷ�����Ӧ�Ļ�ѧ����ʽΪ_____________________����ƽ��ѧ����ʽ�����ù��������е�NaClO3��ClO2��NaClO2����ǿ�����������Ƕ��ܺ�Ũ���ᷴӦ��ȡCl2�����ö������Ⱥ�Ũ������ȡCl2��������5 mol Cl2ʱ��ͨ����ԭ��Ӧ�Ƶ�����������Ϊ________�ˡ�
��2������Һ�еõ���NaClO2��3H2O����IJ���������__________����д��ţ���
a.���� b.���� c.���� d.��ȴ�ᾧ
��3��ӡȾ��ҵ�����������ƣ�NaClO2��Ư��֯�Ư��֯��ʱ���������õ���HClO2���±���25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
�ٳ����£����ʵ���Ũ����ȵ�NaClO2��NaF��NaCN��Na2S������Һ��pH�ɴ�С��˳��Ϊ____________�������ȣ����ʵ���Ũ����ͬ��NaF��NaCN����Һ�������������������Ĵ�С��ϵΪ��______________���ǰ�ߴ���ȡ����ߴ���
��Na2S�dz��õij�������ij��ҵ��ˮ�к��е�Ũ�ȵ�Cu2+��Fe2+��Pb2+���ӣ��μ�Na2S��Һ�����������ij�����_______�������һ�����ӳ�����ȫʱ��������Ũ��Ϊ10-5mol��L-1����ʱ��S2-��Ũ��Ϊ____��
��֪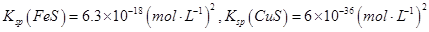
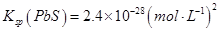
��14�֣���1��2NaOH+2ClO2+H2O2��2NaClO2+2H2O+O2�� 71��ÿ��2�֣���4�֣���2��dc��2�֣�
��3����Na2S��NaCN��NaF��NaClO2 ǰ�ߴ�ÿ��2�֣���4�֣�
��CuS 6.3��10��13mol/L��ÿ��2�֣���4�֣�
���������������1������������Ϣ��֪��������������NaClO2������һ����ClO2��NaClO2�����ϼ۽��ͣ�����ԭ����H2O2�ض�����������������������˷�Ӧ�Ļ�ѧ����ʽΪ2NaOH+2ClO2+H2O2��2NaClO2+2H2O+O2�����������Ⱥ�Ũ������ȡCl2ʱ��������������Ԫ�صĻ��ϼ۴ӣ�4�۽��͵�0�ۣ��õ�4�����ӡ��Ȼ�������Ԫ�صĻ��ϼ۴ӣ�1�����ߵ�0�ۣ�ʧȥ1�����ӣ�����ݵ��ӵĵ�ʧ�غ��֪���������ͻ�ԭ��������ʵ���֮����1:4����ԭ�����������������ʵ���֮��Ҳ��1:4����˵�����5 mol Cl2ʱ��ͨ����ԭ��Ӧ�Ƶ����������ʵ�����1mol������Ϊ71g��
��2������Һ�еõ����ᾧˮ�ľ��壬ֻ�ܲ�ȡ������Ũ������ȴ�ᾧ������ͨ�����˵õ��־��壬���Բ���˳��Ϊdc��
��3������Խ������Ӧ������Խ����ˮ�⣬��Һ�ļ���Խǿ��pHԽ����ĵ���ƽ�ⳣ��ԽС����Խ�������Ը��ݵ���ƽ�ⳣ����֪������ǿ��˳����HClO2��HF��H2S��HCN��HS���������£����ʵ���Ũ����ȵ�NaClO2��NaF��NaCN��Na2S������Һ��pH�ɴ�С��˳��ΪNa2S��NaCN��NaF��NaClO2�����ݵ���غ��֪��NaF��NaCN����Һ�д���c(Na��)��c(H��)��c(OH��)��c(F��)��c(Na��)��c(H��)��c(OH��)��c(CN��)���������Һ�������������������ֱ���n(Na��)��n(H��)��n(OH��)��n(F��)��2 n(Na��)��2n(H��)��n(Na��)��n(H��)��n(OH��)��n(CN��)��2 n(Na��)��2n(H��)�������ʵ���Ũ����ȵ������£�NaF��Һ�ļ�������NaCN��Һ�ļ��ԣ���NaF��Һ�������ӵ�Ũ�ȴ���NaCN��Һ�������ӵ�Ũ�ȣ���˻���ȣ����ʵ���Ũ����ͬ��NaF��NaCN����Һ�������������������Ĵ�С��ϵΪǰ�ߴ�
�ڸ����ܶȻ�������֪��CuS���ܶȻ�������С���������е�Ũ�ȵ�Cu2+��Fe2+��Pb2+���ӵ���Һ�У��μ�Na2S��Һ�����������ij�����CuS�������������FeS����������Һ��c(Fe2+)��10-5mol/Lʱ������FeS���ܶȻ�������֪����Һ��c(S2��)��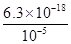 ��6.3��10��13mol/L��
��6.3��10��13mol/L��
���㣺����������ԭ��Ӧ����ʽ����ƽ�����㣻���ʵķ������ᴿ��������ʵĵ��롢����ˮ���Լ���Һ������Ũ�ȴ�С�Ƚϣ��ܶȻ�������Ӧ�úͼ����


 һ����������ϵ�д�
һ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����к͵ζ�������ʵ�������Ź㷺��Ӧ�á�����ʱ����0.250 mol/LNaOH��Һ�ζ�25.0 mL��һԪ��HR��Һʱ����Һ��pH�仯�����ͼ��ʾ������a���ʾ��������ǡ����ȫ��Ӧ����ش��������⣺
��1����һԪ��HR��Һ�����ʵ���Ũ��Ϊ_______________��
��2��ͼ��x_____7���>������<����=��������ԭ����___________________�������ӷ���ʽ��ʾ����
��3���ζ���a��ʱ����Һ��c(OH��)--c(HR)="____mol/L" (�ú�x�Ĵ���ʽ��ʾ)��
��4������ʱ��HR�ĵ��볣��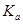 =____________mol/L��
=____________mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ȼ�����Һ�д�������ƽ�⣺FeCl3+3H2O Fe(OH)3+3HC1;��H��0���ش��������⣺
Fe(OH)3+3HC1;��H��0���ش��������⣺
(1)����FeCl3��Һ����Һ����ɫ��ϼ���ɵõ�һ�ֺ��ɫ��Һ�壬������Һ
���м���MgCl2��Һ������������Ϊ ��
(2)���ϼ���FeCl3��Һ��������ˮ�ֲ����յõ��Ĺ�������� ��
(3)������FeCl3��Һʱ��Ϊ��ֹ���ǣ�Ӧ���� ��
(4)Ϊ�˳�ȥMgCl2������Һ�е�Fe3+�����ڼ��Ƚ���������¼���MgCO3���壬����
���ټ����������ᡣMgCO3�����ܳ�ȥFe3+��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����п���Ʊ�ӫ��۵�ԭ��֮һ����ҵ����п����Ҫ�ɷ���ZnO��������Fe2O3��CuO��SiO2�����ʣ��Ʊ�ZnSO4?7H2O���������¡�
��֪�������£���Һ�е�Fe3+��Zn2+��Fe2+������������ʽ��ȫ������pH�ֱ�Ϊ��3.7��6.5��9.7��
��1����ȡ��������߽���Ч�ʿɲ��õĴ�ʩ���������δ�һ������
ZnO�����ᷴӦ�Ļ�ѧ����ʽΪ������
��2����������п�۵�����Ϊ����ʹ��Һ�е�Fe3+ת��ΪFe2+����������
��3������������H2O2������Ӧ�����ӷ���ʽΪ������
��4����������Ca(OH)2������ҺpH���ٽ�Fe3+ˮ�⣬Fe3+ˮ�ⷴӦ��ƽ�ⳣ������ʽ
K��������Ca(OH)2���ܹ�����ԭ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��Ksp(AgCl)=1.8��10��10��Ksp(AgI)=1.5��10��16 ��Ksp(Ag2CrO4)=2.0��10��12��Ag2CrO4Ϊש��ɫ������
(1) AgCl��AgI�� Ag2CrO4���ֱ�����Һ�У�Ag+Ũ���ɴ�С˳���� ���ֽ������2.0��10��4 mol��L��1��AgNO3��Һ��һ��Ũ�ȵ�Na2CrO4��Һ��ϣ���Ҫ����Ag2CrO4��������Na2CrO4��Һ����Ũ��Ϊ mol��L��1��
(2) �������£���0.02mol��L��1��Na2CrO4��Һ�еμ�0.01mol��L��1ϡ���ᣬ��Һ�ɻ�ɫת��Ϊ�Ⱥ�ɫ��Na2Cr2O7����ƽ��ת�������ӷ���ʽΪ ��Na2Cr2O7����������NaCl��NaNO2������ʱ���������ӷ���ʽΪ ��
(3) ��ҵ���Է�ˮ�к�Cr2O72�����ӻ���ɸ���Ⱦ���ŷ�ǰ�Ƚ�Cr2O72����ԭ��Cr3+����ת����Cr(OH)3��ȥ����ҵ�ϲ��õķ��������ˮ�м���NaCl������Ϊ�缫���е�⣬ͬʱ��������������Һ��pHֵ�������ߣ���Һ������ת��Ϊ���ԡ������ϴ��������У�д���������缫��Ӧʽ�� ��Cr2O72��ת��Ϊ���Խϵ͵�Cr3+�����ӷ���ʽΪ�� ��
(4) ��AgNO3��Һ�ζ���Cl������Һ���ɲⶨ��Һ�е�c(Cl��)���ɵ��뼸�� ��ҺΪָʾ�����ζ����յ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��22�֣��о�̼���仯��������ʺ���;����ѧ��ѧ������֮һ��
I��ij��ȤС����ʵ�������Ʊ�̼������Һ���������£�����һ��ȡ25 mLһ��Ũ�ȵ�NaOH��Һ��ͨ��CO2�������������������������в���һ������Һ������������ȡ25 mL��ͬŨ�ȵ�NaOH��Һ�벽���������Һ��ϣ�����̼������Һ��
��1����ɲ���һ��ѡ�Ļ�ѧ�Լ��У�ϡ���ᡢNaOH��Һ������ʯ������̼������Һ��ϡ���ᡢ����̼��������Һ�ȣ���Ҫ��װ��������ʾ��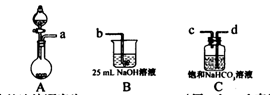
�ٸ�װ����ȷ������˳��Ϊ ����a��b��c��d��ʾ����
�ڼ������װ�������Եķ����� ��
��װ��A�г���ҩƷΪ ��װ��C�л�ѧҩƷ�������� ��
��2��д���������з�����Ӧ�����ӷ���ʽ ��
��3����ͬѧ������������û�б�Ҫ����������Լ��Ŀ��� ��
��ʵ�����ô�������ᴦ��ij������ʯ����֪����ʯ�к���MgO��SiO2��CaO��Fe2O3��Al2O3�������ģ���Ʊ�����þ���������£�
��1��������Ҫ�IJ��������� ��
��2��������ijɷ�Ϊ ���������ӷ���ʽ��ʾ���ɳ����Ĺ��� ��д��һ�����ɣ���
��3��������Һ����������Ũ�ȵĴ�С��ϵΪ ��
��4����֪l0%�Ĵ�����Һ�ܶ�Ϊ1��06g��cm3���������ʵ���Ũ��Ϊ____ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
(14��) CuCl���л��ϳɵ���Ҫ���������������ϡ������ȹ�ҵ����ҵ���ɷ�ͭ�ϣ���Fe��Al���仯���SiO2���ʣ�������CuCl�Ĺ����������£�
| ���� | ��ʼ���� | ������ȫ |
| Fe(OH)3 | 2.7 | 3.7 |
| Cu(OH)2 | 5.6 | 6.7 |
| Al(OH)3 | 3.8 | 4.7 |
 CuOH + H+��ƽ�ⳣ��Ϊ�� ��
CuOH + H+��ƽ�ⳣ��Ϊ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��һ���¶��£����0��1 mol��L��1CH3COOH��Һ��PHΪ3��0����CH3COOH��ˮ�еĵ����Ϊ �����¶�CH3COOH�ĵ���ƽ�ⳣ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���к͵ζ����ⶨij�ռ���Ʒ�Ĵ���,�Ը���ʵ��ش���������:
��1��ȷ����8.2 g�������������������ʵ���Ʒ,���500 mL������Һ?����ʱ,��Ʒ�ɷ�
��________��������ĸ������?
A��С�ձ��� B���ྻֽƬ�� C��������
��2�� �ζ�ʱ,��0.2000 mol��L-1���������ζ�������Һ,����ѡ��____��������ĸ����ָʾ��?
A������ B��ʯ�� C����̪
��3��������̨�ϵ�һ�Ű�ֽ,��Ŀ����_______ _?
��4����0.20 mol��L-1���������ζ�10.00 mL������Һ���ζ���ֹʱ����������Һ20.00mL�����㱻���ռ���Һ�����ʵ���Ũ����________mol��L-1,�ռ���Ʒ�Ĵ�����________?
��5������ʵ�����������ƿ�ô���Һ��ϴ,Ȼ���ټ���10.00 mL����Һ,��ζ���� ���ƫ�ߡ�?��ƫ�͡�����Ӱ�족����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com