
���Ȼ�����Һ�д�������ƽ�⣺FeCl3+3H2O Fe(OH)3+3HC1;��H��0���ش��������⣺
Fe(OH)3+3HC1;��H��0���ش��������⣺
(1)����FeCl3��Һ����Һ����ɫ��ϼ���ɵõ�һ�ֺ��ɫ��Һ�壬������Һ
���м���MgCl2��Һ������������Ϊ ��
(2)���ϼ���FeCl3��Һ��������ˮ�ֲ����յõ��Ĺ�������� ��
(3)������FeCl3��Һʱ��Ϊ��ֹ���ǣ�Ӧ���� ��
(4)Ϊ�˳�ȥMgCl2������Һ�е�Fe3+�����ڼ��Ƚ���������¼���MgCO3���壬����
���ټ����������ᡣMgCO3�����ܳ�ȥFe3+��ԭ���� ��
��1�����ɺ��ɫ���� ��2��Fe2O3 (3)Ũ����
��4��MgCO3��Fe3+ˮ�������H+��Ӧ���ٽ���Fe3+ˮ�⣬ʹFe3+ת��ΪFe��OH��3��������ȥ��
�������������(1)MgCl2�ǵ���ʣ���ʹ����۳�,���Ի�������ɫ������
(2)Fe(OH)3ʧˮ�õ�Fe2O3��
(3)��FeCl3��Һ�м���Ũ����ʹFe3+ˮ��ƽ�����ƣ���ֹ��ӦFeCl3+3H2O Fe(OH)3+3HC1����������Fe(OH)3��
Fe(OH)3+3HC1����������Fe(OH)3��
(4)�Ȼ�����̼��þ��ˮ�⻥��ٽ�����MgCO3��Fe3+ˮ�������H+��Ӧ���ٽ���Fe3+ˮ�⣬ʹFe3+ת��ΪFe��OH��3��������ȥ��
���㣺���⿼����ˮ�⣬�����ܽ�ƽ������֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
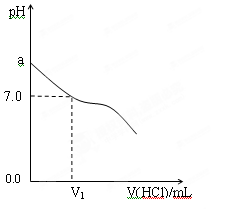
��1�������£���NH3��H2O������������ϣ�ʵ���������£�
| ��� | NH3��H2O | HCl | �����Һ��pHֵ |
| �� | c��NH3��H2O��=0.1mol��L-1 | c��HCl��=0.1mol��L-1 | pH=a |
| �� | NH3��H2O��pH=12 | HCl��pH=2 | pH=b |
| �� | c��NH3��H2O��="A" mol��L-1 | c��HCl��=0.1mol��L-1 | pH=c |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����л�Ϻ���Һ��pH����lg2=0.3 lg5=0.7��
��1����pH=2��pH=4������ǿ����Һ�������ϣ���pH=_______��
��2����pH=12��pH=14������ǿ����Һ�������ϣ���pH=_________��
��3����pH=2��H2SO4��Һ��pH=10��NaOH��Һ�������ϣ���pH=_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʢ�Cu����Һ̬SO2����CH3COOH����NaHCO3����Ba(OH)2��Һ��������NaCl����ϡ��ˮ����BaSO4����H2O
��1������ǿ����ʵ��� ������ţ� ����������ʵ��� ������ţ�
��2�������£�0.1 mol��L-1NaHCO3��Һ��pH����8������Һ��Na����HCO3�D��CO32�D��OH�D��������Ũ���ɴ�С��˳��Ϊ�� ��NaHCO3ˮ������ӷ���ʽ ��
��3��Ba(OH)2��һ��ǿ����ʣ�����25�桢pH��13��Ba(OH)2��Һ��
�ٸ�Ba(OH)2��Һ�����ʵ���Ũ��Ϊ___________________��
����ijŨ��������Һ������ȣ�������֮�ȣ�1 ��9��Ϻ�������ҺpH��11����������Һ��������ڻ��ǰ����Һ������ͣ�����������Һ��pH��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ����ģ�����ij�Ͼɺ�����������Ҫ�ɷ�ΪNiO������Fe2O3��CaO��CuO��BaO�ȣ�����Ni2O3���乤������Ϊ��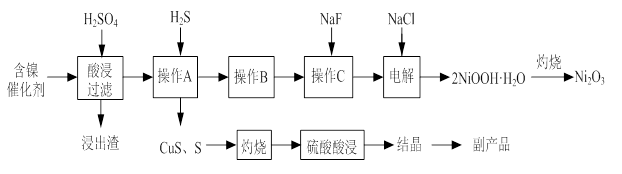
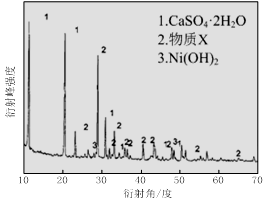
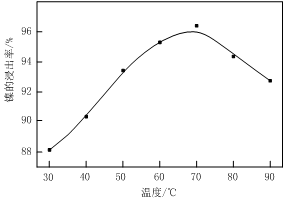
ͼ�� ͼ��
��1������ͼ����ʾ��X��������ͼ�ף���֪����������������Ҫ�ɷ֣����С�����X��Ϊ ��ͼ���ʾ���Ľ��������¶ȵĹ�ϵ���������¶ȸ���70��ʱ�����Ľ����ʽ��ͣ���������Ni(OH)2����������ԭ���� ��
��2�����������С�����Ʒ���Ļ�ѧʽΪ ��
��3����֪�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Fe(OH)3 | Fe(OH)2 | Ni(OH)2 |
| ��ʼ������pH | 1.5 | 6.5 | 7.7 |
| ������ȫ��pH | 3.7 | 9.7 | 9.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����һ�仰������������ֵ��ǰ��ΪM������ΪN��M��N�Ĺ�ϵ��A��B��C��D ��ѡ��
A��M>N B��M<N C�� M="N" D�� ���Ƚ�
��1����ͬ�¶��£�1L 1mol/L ��NH4Cl��Һ��2 L 0.5mol��L��1NH4Cl��Һ������Һ��NH4+�ĸ����� ��
��2����ͬ�¶��£�pHֵΪ12���ռ���Һ��CH3COONa��Һ������Һ��ˮ�ĵ���̶ȣ� ��
��3�����������ݵ�Ũ�ȵĴ�����Һ�����ڶ��������¶ȣ�����Һ��c(HCO3��)�� ��
��4����pHֵΪ2�Ĵ�������ᶼϡ����ͬ��������ϡ��Һ��pHֵ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������ѧ��ѧ�г������ᣬ��һ�������£�CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH CH3COO-+H+ ��H��0��
CH3COO-+H+ ��H��0��
��1�������£��� pH =5��ϡ������Һ�У�c(CH3COO-)��____________(��ʽ�����ػ���)�����з����У�����ʹ0��10 mol��L-1 CH3COOH�ĵ���̶��������______?
a����������0��10 mol��L-1��ϡ���� b������CH3COOH��Һ
c����ˮϡ����0��010 mol��L-1 d����������������
e�����������Ȼ��ƹ��� f����������0��10 mol��L-1��NaOH��Һ
��2������������пͶ��������pH������3�Ĵ����������Һ�У�������ַ�Ӧ����ֻ��һ����Һ����п��ʣ�࣬�����������������V(����)_________V(����)����Ӧ���������Ϊ��v(����)_________v(����)��(��д������������������)
��3��ijͬѧ��0��1000mol/LNaOH��Һ�ֱ�ζ�20��00mL 0��1000mol/LHCl��20��00mL0��1000mol/L CH3COOH���õ���ͼ��ʾ�����ζ����ߣ�������й����⣺
��NaOH��Һ�ζ�CH3COOH��Һ�������� ���ͼ1����ͼ2������
��a�� mL��
��4�������£���0��1 mol/L�����0��1 mol/L��������Һ��ϣ�������ҺΪ���ԣ�������Һ�и����ӵ�Ũ�Ȱ��ɴ�С����Ϊ_______________________________��
��5����֪��90��ʱ��ˮ�����ӻ�����ΪKw = 3��80��10-13���ڴ��¶��£���pH=3�������
pH = 11������������Һ�������ϣ�������Һ�е�c(H+)=____________(������λ��Ч����)mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ƣ�NaClO2��������ˮ��������ɰ�ǡ���֬��Ư����ɱ�����������ù������ⷨ�����������ƵĹ�������ͼ��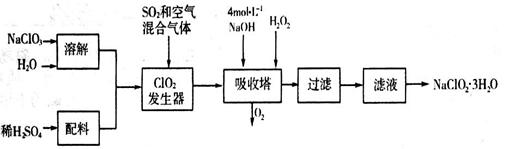
��֪��NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
��1���������ڷ�����Ӧ�Ļ�ѧ����ʽΪ_____________________����ƽ��ѧ����ʽ�����ù��������е�NaClO3��ClO2��NaClO2����ǿ�����������Ƕ��ܺ�Ũ���ᷴӦ��ȡCl2�����ö������Ⱥ�Ũ������ȡCl2��������5 mol Cl2ʱ��ͨ����ԭ��Ӧ�Ƶ�����������Ϊ________�ˡ�
��2������Һ�еõ���NaClO2��3H2O����IJ���������__________����д��ţ���
a.���� b.���� c.���� d.��ȴ�ᾧ
��3��ӡȾ��ҵ�����������ƣ�NaClO2��Ư��֯�Ư��֯��ʱ���������õ���HClO2���±���25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
�ٳ����£����ʵ���Ũ����ȵ�NaClO2��NaF��NaCN��Na2S������Һ��pH�ɴ�С��˳��Ϊ____________�������ȣ����ʵ���Ũ����ͬ��NaF��NaCN����Һ�������������������Ĵ�С��ϵΪ��______________���ǰ�ߴ���ȡ����ߴ���
��Na2S�dz��õij�������ij��ҵ��ˮ�к��е�Ũ�ȵ�Cu2+��Fe2+��Pb2+���ӣ��μ�Na2S��Һ�����������ij�����_______�������һ�����ӳ�����ȫʱ��������Ũ��Ϊ10-5mol��L-1����ʱ��S2-��Ũ��Ϊ____��
��֪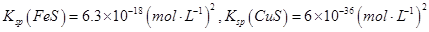
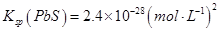
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�Ǒz�ᣨH3PO3)�Ƕ�Ԫ�ᣬ������NaOH��Һ��Ӧ����Na2HPO3��
��1��PCl3ˮ�����ȡ�����PCl3+3H2O=H3PO3+_______��
��2��H3PO3��Һ�д��ڵ���ƽ�⣺H3PO3 H++H2PO3����
H++H2PO3����
��ij�¶��£�0.10mol?L��1�� H3PO3��Һ pH =1.6������Һ��c(H+) =2.5��10��2 mol?L��1������¶�����������ƽ���ƽ�ⳣ��K��д��������̡���H3PO3�ĵڶ���������Բ��ƣ����������λ��Ч���֡���
�ڸ���H3PO3�����ʿ��Ʋ�Na2HPO3ϡ��Һ��pH________7 (�>������=����<������
��3�����������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ_______��
��4�����Na2HPO3��ҺҲ�ɵõ����[�ᣬװ��ʾ��ͼ���£�
�������ĵ缫��ӦʽΪ____________________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ_____________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com