
����Ŀ��ʵ�������ܶ�Ϊ1.25 g��mL��1����������Ϊ36.5%��Ũ���ᣬ����240mL0.1mol��L��1�����ᣬ��ش��������⣺
��1��Ũ��������ʵ���Ũ��Ϊ_____��
��2������0.1 mol��L��1������Ӧ����Ͳ��ȡŨ�������____mL��
��3������ʱ������ȷ�IJ���˳����(����ĸ��ʾ��ÿ����ĸֻ����һ�Σ�������ȱ�������ں�����)______��
A����30 mL����ˮϴ��____ 2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ�����Ũ�����������ز����������ձ��У��ټ�������ˮ(Լ30 mL)���ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע������ƿ��
D��������ƿ�ǽ�����ҡ��
E������ _____��ˮ��ʹ��Һ��Һ��ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���____��
��4����ʵ������г������������������ҺŨ�Ƚ�������������ƫ��ƫ�ͣ�
�ټ�����ˮʱ���������̶���_____����ȡŨ����ʱ����______�۶���ʱ����_______
���𰸡�12.5mol/L 2.0 BCAFED �ձ��ڱںͲ����� ��ͷ�ι� 1~2cm ƫ�� ƫ�� ƫ��
��������
(1)����c=![]() ����Ũ��������ʵ���Ũ�ȣ�
����Ũ��������ʵ���Ũ�ȣ�
(2)������Һϡ��ǰ���������ʵ����ʵ������������ҪŨ����������
(3)������Ũ��Һ����ϡ��Һ��һ�����������
(4)�������������ʵ����ʵ�������Һ�����Ӱ�죬�����жϡ�
(1)Ũ��������ʵ���Ũ��c=![]() =12.5mol/L��
=12.5mol/L��
����: 12.5mol/L��
(2)Ҫ����240mL����Һ��Ӧѡ��250mL������ƿ������ҪŨ��������ΪV��������Һϡ��ǰ���������ʵ����ʵ��������V��12.5mol/L=250mL��0.1mol��L��1������ó�V=2.0mL��
����: 2.0��
(3)Ũ��Һ����ϡ��Һ��һ��������裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�������Ϸ�����֪������ʱ������ȷ�IJ���˳����BCAFED�������ձ��ڱ���մ�����ʣ�Ϊ��ֹ����������ϴ���ձ��ڱںͲ����� 2��3�Σ�����ʱ����ʼʱֱ�Ӽ�������ˮ��Ϊ��ֹ��ˮ����������̶���1-2cmʱ�����ý�ͷ�ι���εμӵ���Һ����ʹ���̶������У�
�ʴ��ǣ�BCAFED���ձ��ڱںͲ���������ͷ�ιܣ�1~2cm��
(4) �ټ�����ˮʱ���������˿̶��ߣ������Һ���ƫ����c=![]() ��֪��������Һ��Ũ��ƫ�ͣ�
��֪��������Һ��Ũ��ƫ�ͣ�
����ȡŨ����ʱ���ӣ�������ʵ������࣬����c=![]() ��֪��������Һ��Ũ��ƫ�ߣ�
��֪��������Һ��Ũ��ƫ�ߣ�
�۶���ʱ���ӣ���Һ�����ƫС������c=![]() ��֪��������Һ��Ũ��ƫ�ߣ�
��֪��������Һ��Ũ��ƫ�ߣ�
�ʴ��ǣ�ƫ�ͣ�ƫ�ߣ�ƫ�ߡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʵ����Ǹ��л�ѧ���õ�������������������йؼ��㣺
��1��0.25 mol CH4�ڱ�״���µ����Ϊ_______L��0.25 mol CH4�к���______����ԭ�ӡ�
��2����ͬ������CO��CO2������������ԭ����֮��Ϊ_______����״���£�������ͬ��ԭ������CO��CO2�����֮��Ϊ________��
��3����9.5 gij���۽������Ȼ����к���0.2 mol Cl-�����Ȼ����Ħ������Ϊ________���ý���Ԫ�ص����ԭ������Ϊ__________��
��4����agij�����к��еķ�����Ϊb����cg�������ڱ�״���µ������___________(��NAΪ�����ӵ�������ֵ)��
��5����״�����Т�0.112 Lˮ����0.5NA��HCl���Ӣ�25.6 g SO2���塡��0.2 mol����(NH3)����2 mol Ne����ԭ�Ӹ����Ӵ�С��˳��Ϊ_____��
��6��6.72 L CO(��״��)��һ������Fe2O3ǡ����ȫ��Ӧ(����Fe��CO2)������Fe������Ϊ__________g��
��7��ij�¶�ʱ��һ������Ԫ��A����̬�⻯��AH3���ں����ܱ������зֽ�Ϊ�������嵥�ʣ���ʱѹǿ������75������AH3�ֽⷴӦ�Ļ�ѧ����ʽ________________________��
��8��V mL Fe2(SO4)3��Һ�У�����Fe 3+ m g��ȡ��V/2mL����Һϡ����4V mL������Һ��SO42-�����ʵ���Ũ��Ϊ___________�����ú���m��V��ʽ�ӱ�ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܸ߷���P�ĺϳ�·����ͼ��

��1��A�ķ���ʽ��C7H8����ṹ��ʽ��_____��
��2���Լ�a��____��
��3����Ӧ�۵Ļ�ѧ����ʽ��___��
��4��������H����A��ͬϵ������ʽΪC8H10����ͬ���칹�干��___�֣�����___(д�ṹ��ʽ)��һ�ȴ���ֻ�����֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��֪:H2(g)+1/2O2(g)H2O(g),��Ӧ�����������仯��ͼ��ʾ,��:
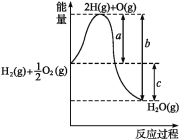
��a��b��c�ֱ����ʲô����?
a______________________________;
b_______________________________;
c_______________________________��
�ڸ÷�Ӧ����H____________0(����>������<��)��
(2)�������칬��һ�ŵĻ��ʹ�õ��ƽ�����Һ���Һ��,��֪:H2(g)+![]() O2(g)H2O(l)����H=-285.8 kJ��mol-1
O2(g)H2O(l)����H=-285.8 kJ��mol-1
H2(g)H2(l)����H=-0.92 kJ��mol-1
O2(g)O2(l)����H=-6.84 kJ��mol-1
H2O(l)H2O(g)����H=+44.0 kJ��mol-1
��д��Һ���Һ��������̬ˮ���Ȼ�ѧ����ʽ____________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�һ���ܱ��������м���һ�������ɻ����ĸ��壨��ȿɺ��ԣ��������ֳ������֣�����߳���������ұ߳���H2��O2�Ļ�����壬����ǡ�ô��������м�λ�ã���H2��O2�Ļ�������ȼ���������������ָ������º���ͣ����ͼλ�á���ԭ��H2��O2�����ʵ���֮�ȿ���Ϊ

��4:1 �� 2:1 ��1:1 ��2:3
A.�٢�B.�٢�C.�ڢ�D.�ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������(AlP)��һ�ֳ�������ʳ�ִ��Ĺ�����Ѭ��ɱ�������ˮ���������߶�������PH3(�е㣭89.7�棬��ԭ��ǿ)����������ί�涨��ʳ������(��PH3��)�IJ�����������0.05mg/kgʱΪ�����ϸ�֮���ϸ�ij��ѧ��ȤС���ͬѧ�����������ⶨij��ʳ��Ʒ�в���������������ж��Ƿ�ϸ�
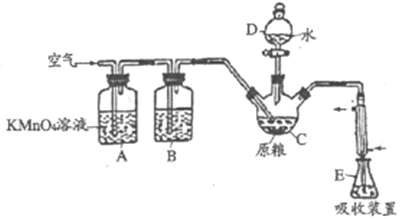
��C�м���100gԭ����E�м���20.00mL2.50��10��4mol/ L KMnO4��Һ(H2SO4�ữ)����C�м�������ˮ����ַ�Ӧ�����������Ʊ���Һ�ζ�E�й�����KMnO4��Һ���ش��������⣺
(1)PH3�ĵ���ʽΪ___________������D��������___________��
(2)ͨ�������������___________��
(3)װ��B��ʢװ����ûʳ����ļ�����Һ�������������տ����е�O2����ȥ����װ�ã���ʵ���Ӱ��Ϊ___________��
(4)װ��E��PH3�����������ᣬ��װ��E�з�����Ӧ�����ӷ���ʽΪ__________���ռ�װ��E�е�����Һ����ˮϡ����250mL��ȡ25.00mL����ƿ�У���4.0��10��5mol/L��Na2SO3����Һ�ζ�ʣ���KMnO4��Һ������Na2SO3����Һ20.00mL��Na2SO3��KMnO4��Һ��Ӧ�����ӷ���ʽΪ��SO32��+MnO4��+H+��SO42��+Mn2++H2O(δ��ƽ)�����ԭ����Ʒ������(��PH3��)������Ϊ__________mg����ԭ����Ʒ__________(�����ϸ����������ϸ���)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ͼ������˵����ȷ����

A. ��ͼ��֪����Ӧ�� T1��T3 ���ﵽƽ��
B. ��ͼ��֪���÷�Ӧ�ġ�H��0
C. ��ͼ��֪��t3ʱ��ȡ���ͷ�Ӧ�¶ȵĴ�ʩ
D. ��ͼ��֪����Ӧ�� t6ʱ��NH3 ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶���,����̼����MCO3��M�ֱ�ΪA��B�������ӣ��ij����ܽ�ƽ��������ͼ��ʾ����֪��pM=lgc(M)��p(CO32)=lgc(CO32)����֪ACO3��BCO3�ܽ�ȸ����������������ӵ�ˮ�⣩������˵����ȷ����

A. ��a��ʾACO3���ܽ�ƽ������
B. ���¶��£���ACO3�ı�����Һ�м���Na2CO3��Һ��һ���ܲ�������
C. ��0.1L 1mol/L��BCl2��Һ�м���Na2CO3���壬������Na2CO3���������Ϊ116.6gʱ��B2+����ǡ�ó�����ȫ��B2+����Ϊ10��5mol/Lʱ��Ϊ������ȫ��
D. ACO3��Ksp=10��4.4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͬ�����£��������ʷֱ���H2��Ӧ�������ĵ����ʵ���������ʱ���ų������������ǣ� ��
A. Cl2B. Br2C. I2D. F2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com