
����Ŀ��[��ѧһѡ��3: ���ʽṹ������]
A��B��C��DΪԪ�����ڱ���ǰ�����ڵ�����Ԫ�أ���ԭ��������������C��Dͬ���ڣ���A��B��C������ͬһ���ڣ�����A��Dͬ���壬�Ҹ������������ַǽ���Ԫ�أ�BΪ�ǽ���Ԫ����ԭ���������3�ԳɶԵ��ӣ�CԪ��λ��Ԫ�����ڱ���10�С���ش���������:
(1)CԪ�ص�ԭ������Ϊ________����̬Dԭ�ӵļ����Ų�ʽΪ________��
(2)��Aͬһ���ڵ���������Ԫ���е�һ������С��A��Ԫ�ع���_______�֡�
(3) DԪ�ؿ��γ�DX3��±������ʺͽṹ��AX3����(X��ʾ±��Ԫ��)����ˮ��Һ��ǿ��ˮ�⣬��д��DCl3��ˮ��Ӧ�Ļ�ѧ����ʽ:____________��
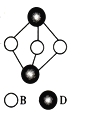
(4) ��ͼΪD2B3�ķ��ӽṹͼ��B�ӻ���ʽΪ____________��
(5) �ԱȽ�A��B�γɵļ��⻯����۷е�ĸߵͲ����ͣ�________ (���⻯���÷���ʽ��ʾ)��
(6) �о�����ṹ����÷�����________����ͼΪһ�ֺ�C��D����Ԫ����Ʒ��ľ���ͼ���������ṹ����������Ϊ����߳�Ϊanm����Ϊbmm��
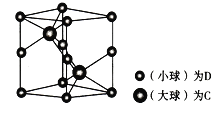
�ٸþ���������ʵĻ�ѧʽΪ__________��
����֪D��C�����ԭ�������ֱ�ΪM1��M2,�������ܶ�Ϊ��g/cm3,NA��ʾ�����ӵ��������ú�������ʽ��ʾ�þ�����ܶ�Ϊ________g/cm3 (�г�����ʽ���ɣ����ػ���)��
���𰸡� 28 [Ar]3d104s24p3 5 AsCl3+3H2O=H3AsO3+3HCl sp3�ӻ� NH3��HCl��NH3���ڷ��Ӽ������HCl���Ӽ�ֻ���ڷ��»���������������ȷ��»���ǿ��NH3���۷е����HCl X-�������� NiAs��AsNi 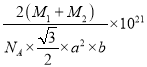
��������������������⿼�����ʽṹ�����ʣ��漰Ԫ�ص��ƶϣ������Ų�ʽ����д����һ�����ܵıȽϣ��ӻ���ʽ���жϣ��۷е�ߵ͵ıȽϣ������ķ����ͼ��㡣C��ǰ������Ԫ�أ�CԪ��λ��Ԫ�����ڱ���10����CΪNiԪ����Ni���ڵ������ڣ�C��Dͬ���ڣ�DҲ���ڵ������ڣ�A��B��C��D��ԭ��������������A��B��C������ͬһ���ڣ�A��Dͬ�����Ҹ������������ַǽ���Ԫ�أ�AΪNԪ�أ�DΪAsԪ�أ�B���ڵ���������BΪ�ǽ�����ԭ���������3�ԳɶԵ��ӣ�BΪClԪ����
��1��CΪNiԪ����Ni��ԭ������Ϊ28��DΪAs��As��ԭ������Ϊ33�����ݹ���ԭ������̬As�ĺ�������Ų�ʽΪ1s22s22p63s23p63d104s24p3�������Ų�ʽΪ[Ar] 3d104s24p3��
��2��AΪN��N���ڵڶ����ڣ��ڶ������е�һ������С��N��Ԫ����Li��Be��B��C��O����5����
��3��AsCl3��ˮ��Һ��ǿ��ˮ������H3AsO3��HCl����Ӧ�Ļ�ѧ����ʽΪAsCl3+3H3O=H3AsO3+3HCl��
��4����ʾ��ͼ�ɼ�B�γ�2���Ҽ���B�ϻ������Թµ��Ӷԣ�B�ļ۲���Ӷ���Ϊ4��B���ӻ���ʽΪsp3�ӻ���
��5��A�ļ��⻯��ΪNH3��B�ļ��⻯��ΪHCl������NH3���ڷ��Ӽ������HCl���Ӽ�ֻ���ڷ��»���������������ȷ��»���ǿ��NH3���۷е����HCl��
��6���о�����ṹ��õķ�����X-�������䡣
��a��������̯������DΪAs��8![]() +4
+4![]() =2��CΪNi��Cȫ�ھ����ڣ�Ni��2��Ni��As�ĸ�����Ϊ2:2=1:1���þ���Ļ�ѧʽΪNiAs��AsNi��
=2��CΪNi��Cȫ�ھ����ڣ�Ni��2��Ni��As�ĸ�����Ϊ2:2=1:1���þ���Ļ�ѧʽΪNiAs��AsNi��
��b���þ����������ṹ�������ĵ����Ϊa![]() 10-7cm
10-7cm![]() a
a![]() 10-7cm=
10-7cm=![]() a2
a2![]() 10-14cm2�����������Ϊ
10-14cm2�����������Ϊ![]() a2
a2![]() 10-14cm2
10-14cm2![]() b
b![]() 10-7cm=
10-7cm=![]() a2b
a2b![]() 10-21cm3��1mol��������Ϊ
10-21cm3��1mol��������Ϊ![]() a2b
a2b![]() 10-21cm3
10-21cm3![]() 2
2![]() NA������Ļ�ѧʽΪNiAs��AsNi��1mol���������Ϊ��M1+M2��g��������ܶ�=
NA������Ļ�ѧʽΪNiAs��AsNi��1mol���������Ϊ��M1+M2��g��������ܶ�=![]() =��M1+M2��g
=��M1+M2��g![]() ��
��![]() a2b
a2b![]() 10-21cm3
10-21cm3![]() 2
2![]() NA��=
NA��=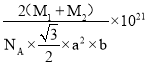 g/cm3��
g/cm3��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������β����Ҫ����CO2 ��CO��SO2��NOx �����ʣ�β����CO����������(NOx) ����Ӱ�����ǵ�����ͽ�������ѧ�����߶Ե�������Ĵ������˹㷺��������о���
��1�����ü��黹ԭNOx
��CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g)��H1=-574kJ/mo l��
��CH4(g)+4NO(g)=2N2(g)+CO2(g)+H2O(g) ��H2=-1160kJ/mo l,
����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ________________________________��
��2������ͬ����CO(g)��H2O(g)�ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У����з�ӦCO(g)+H2O(g)![]() CO2(g)+H2(g)���õ������������ݣ�
CO2(g)+H2(g)���õ������������ݣ�
ʵ���� | �¶��� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
CO | H2O | H2 | CO | |||
1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
2 | 900 | 2 | 1 | 0.4 | 1.6 | 4 |
3 | 900 | a | b | c | d | t |
��ʵ��1����v(CO2) ��ʾ�ķ�Ӧ����Ϊ/span>_________(������λ��Ч���֣���ͬ)��
���÷�ӦΪ________(��������������������) ��Ӧ��ʵ��2�����µ�ƽ�ⳣ��K=________��
������ƽ��״̬ʱ��ʵ��2 ��ʵ��3�и����ʵ����������ֱ���ȣ���t��4min,��a��bӦ����Ĺ�ϵ��_________________________(�ú�a��b����ѧʽ��ʾ)��
��3��CO�����ǵĴ������ɲⶨ����β���Ƿ�����ŷű����÷����ǵĹ���ԭ��������ȼ�ϵ�أ����е������������(Y2O3) �������(ZrO2) ���壬�ܴ���O2-�����ĵ缫��ӦʽΪ____________��
��4��SO2���øƻ�����Ӧ��ȥ����Ӧ���ɵ�CaSO4��һ�������ʣ���Ksp=9.0��10-6������Ũ��Ϊ2��10-3mol/L��Na2SO4��Һ��������CaCl2��Һ����������ɳ�������CaCl2��Һ����СŨ��Ϊ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1869�꣬������ѧ���Ž��з��������˵�һ��Ԫ�����ڱ�����ʾ�˻�ѧԪ�ؼ��������ϵ����Ϊ��ѧʷ�ϵ���Ҫ��̱�֮һ�������й�Ԫ�����ڱ���˵����ȷ���ǣ� ��
A.Ԫ�����ڱ���Ԫ���������ǵڢ�B��
B.Ԫ�����ڱ���18����
C.�ڢ�A���Ԫ��ȫ���ǽ���Ԫ��
D.��������ָ��һ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ����Դ��������Ҫ�ɷ���CH4����״���£�0.5 mol CH4��ȫȼ������CO2��H2Oʱ���ų�445 kJ�����������ʾCH4ȼ���ȵ��Ȼ�ѧ����ʽ��ȷ����( )
A. ![]() CH4(g)��O2(g)
CH4(g)��O2(g) ![]() CO2(g)��H2O(l) ��H =445 kJ/mol
CO2(g)��H2O(l) ��H =445 kJ/mol
B. CH4��2O2CO2��2H2O ��H=890 kJ/mol
C. CH4(g)��2O2(g)CO2(g)��2 H2O(g) ��H =890 kJ/mol
D. CH4(g)��2O2(g)CO2(g)��2H2O(l) ��H = -890 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͬŨ�ȵ�NaCl��MgCl2��AlCl3��Һ���ֱ���AgNO3��Һ��Ӧ�������ɵ�AgCl����������֮��Ϊ3��2��1ʱ��������Һ�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ȤС�������ͼ��ʾװ�ý���ԭ���ԭ����̽������������������ǣ� ��
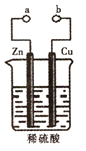
A. a��b�õ�������ʱ��������Zn����������Cu���پ�ϡ��������Zn
B. a��b�õ�������ʱͭƬΪ�����������ķ�ӦʽΪ��2H++2e-=H2��
C. a��b������ʱ��пƬ�����������ɣ���װ�ò����γ�ԭ���
D. ����a��b�Ƿ����ӣ�ϡ��������뷴Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ֻҪ��һ���Լ���������NaCl��MgSO4��FeCl2��FeCl3������Һ�������Լ��ǣ� ��
A.HCl
B.NaOH
C.AgNO3
D.BaCl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ����pH��ֲ����˵ĵ���ø���Ե�Ӱ�죻��ͼ��ʾ����3������ø(A��B��C)�Ļ������¶�Ӱ������������������ȷ����(����)

A. �Ӽ�ͼ�п���֪��pH=6ʱֲ�����ø�Ļ������
B. ����ͼ����֪��øC�������¶�
C. �Ӽ�ͼ�п���֪����ϸ�������Ա�ɼ���ʱ,����ø�Ļ���������
D. ����ͼ�п���֪��ø�����¶ȷ�Χ������B
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
A��B��D��E��FΪԭ���������������ǰ������Ԫ�أ�����A�����������������ڲ��������2����B��D��EΪͬ����Ԫ�أ�Bԭ�ӵĺ��������������δ�ɶԵ�������5����Eԭ���������1��δ�ɶԵ��ӣ�Fԭ�Ӻ�����22���˶�״̬�ĵ��ӡ���ش��������⣺
(1)FԪ��λ�����ڱ�___________������۵����Ų�ͼΪ��___________��
(2)B��D��E����Ԫ���У���һ��������С����_______________________ (��Ԫ�ط���)��д��AD2�ĵȵ�����___________ (���Ӻ������Ӹ�дһ��)��
(3)AO2��DO2�۵�ߵ���___________��ԭ����___________��
(4)B�ĵ��ʺ�E�ĵ��ʷ�Ӧ���γ�ԭ�Ӹ�����Ϊ1��3�Ļ�����M��M�����幹��Ϊ___________������ԭ�ӵ��ӻ��������Ϊ___________��
(5)Ԫ��F������ͬ�������壬�������������ѻ��������������������ѻ�����ͼ��ʾ��F�����һ�־�������������a=0.295nm��c=0.469nm�����F������ܶ�Ϊ___________g��cm-3(��NA��ʾ�����ӵ�������ֵ���г�����ʽ����)��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com